用于生物制药的细胞工程和分子制药。
Q2 Pharmacology, Toxicology and Pharmaceutics
引用次数: 0
摘要
生物制药通常由重组大肠杆菌或哺乳动物细胞系生产。这通常是通过将编码相关蛋白质的基因或 cDNA 导入特性良好的生产细胞株来实现的。当然,每种重组生产系统都有其独特的优缺点。本文探讨了生物制药生产的当前实践、发展和未来趋势。本文回顾了用于快速筛选和分析生物系统的平台技术。此外,还重点介绍了通过代谢工程和综合工程提高生产率的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
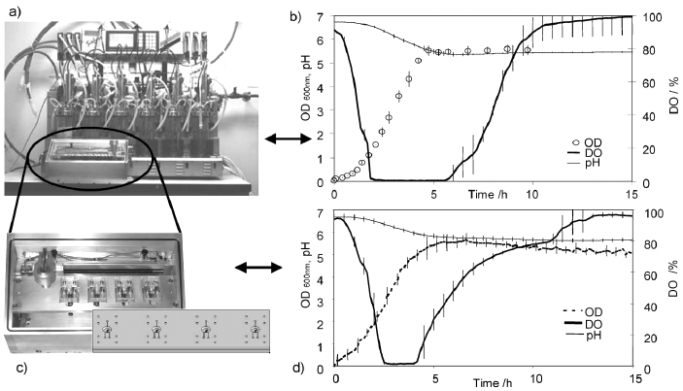
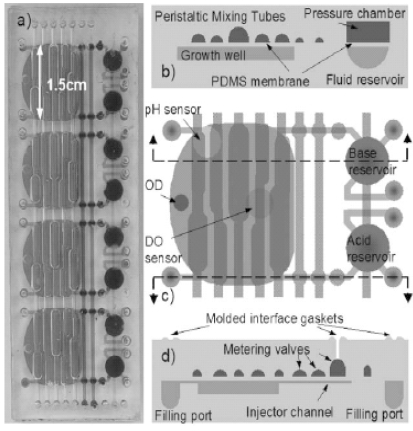
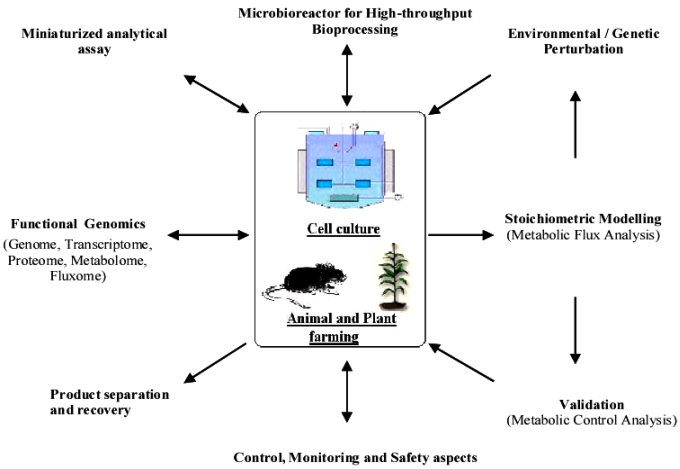
Cell engineering and molecular pharming for biopharmaceuticals.
Biopharmaceuticals are often produced by recombinant E. coli or mammalian cell lines. This is usually achieved by the introduction of a gene or cDNA coding for the protein of interest into a well-characterized strain of producer cells. Naturally, each recombinant production system has its own unique advantages and disadvantages. This paper examines the current practices, developments, and future trends in the production of biopharmaceuticals. Platform technologies for rapid screening and analyses of biosystems are reviewed. Strategies to improve productivity via metabolic and integrated engineering are also highlighted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Open Medicinal Chemistry Journal
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
4.40
自引率
0.00%
发文量
4
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


