IDEAL 作为设计符合新欧洲医疗器械法规的临床器械研究的指南。
IF 2.1
Q2 SURGERY
BMJ Surgery Interventions Health Technologies
Pub Date : 2021-03-04
eCollection Date: 2021-01-01
DOI:10.1136/bmjsit-2020-000066
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
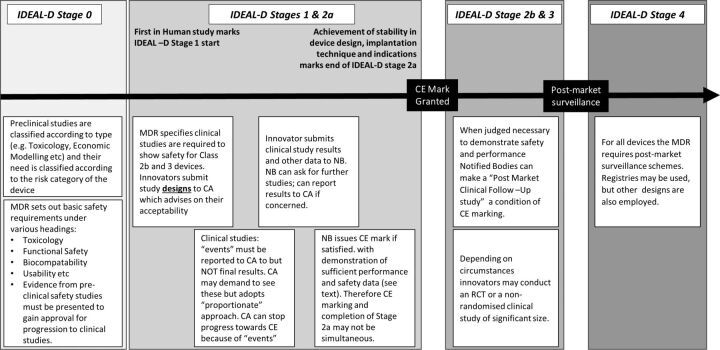
IDEAL as a guide to designing clinical device studies consistent with the new European Medical Device Regulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

BMJ Surgery Interventions Health Technologies
Medicine-Surgery
CiteScore
2.80
自引率
0.00%
发文量
22
审稿时长
17 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


