多金属氧酸盐和金属有机骨架之间电子转移的反应性和稳定性协同作用
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
多金属氧酸盐(POM)和Cu(ii)离子在均相好氧硫醇氧化脱臭中的协同作用已经在一种更实用的非均相催化剂中实现:在金属有机骨架(MOF)的孔隙中捕获具有多电子能力的POM, HKUST-1 (POM@HKUST)。POM与MOF中Cu(ii)节点之间的协同作用取决于POM的类型。磷钒钼酸盐,PVxMo12−xO40(3+x)−(x = 1-3) (PVMo),而不是过渡金属取代的多钨酸盐PXW11 (x = V, Co, Zn和Co),导致POM@MOF材料表现出相对于单个结构组分,POM或MOF单独的协同作用,不仅在类似的均相催化剂的情况下具有反应性,而且具有催化剂结构稳定性。PVMo@HKUST-catalyzed反应进行到基本100%转化,反应后的FTIR光谱、粉末XRD数据等观察表明,材料是可回收且不变的。PXW11@HKUST材料只产生有限的转化,反应后分解成白色粉末。x射线光电子能谱显示HKUST-1中的所有Cu(ii)位都变成了在空气中稳定的Cu(i)位。进一步的动力学研究表明,PVMo与中间Cu/RSH配合物发生快速的多电子转移,而PXW11与这些Cu/RSH配合物的电子转移能力要慢得多,而且有限。Cu节点和封装的POM单元之间有限的电子转移不仅阻碍了反应性,而且导致MOF框架扭曲和随后的分解,这是由框架中Cu(ii)还原到Cu(i)位点引起的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
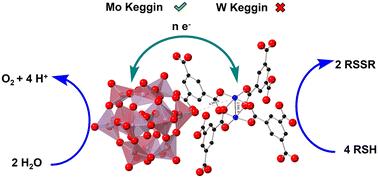
Reactivity and stability synergism directed by the electron transfer between polyoxometalates and metal–organic frameworks†
The synergism between polyoxometalates (POM) and Cu(ii) ions in homogeneous aerobic thiol oxidative deodorization has been realized in a more utilitarian heterogeneous catalyst: a multi-electron-capable POM captured in the pores of a metal–organic framework (MOF), HKUST-1 (POM@HKUST). The synergism between POM and the Cu(ii) nodes in the MOF depends on the type of POM. Phosphovanadomolybdates, PVxMo12−xO40(3+x)− (x = 1–3) (PVMo) but not transition-metal-substituted polytungstates PXW11 (X = V, Co, Zn and Co) result in POM@MOF materials that exhibit synergy relative to the individual structural components, the POM or MOF alone, not only for reactivity as in the case for the analogous homogeneous catalysts, but also for catalyst structural stability. The PVMo@HKUST-catalyzed reaction proceeds to essentially 100% conversion and the material is recoverable and unchanged based on FTIR spectroscopy, powder XRD data and other observations after reaction. The PXW11@HKUST materials produce only limited conversions and decompose to white powders after reaction. X-ray photoelectron spectroscopy reveals that all the Cu(ii) sites in the HKUST-1 become Cu(i) sites that are stable in air. Further kinetics studies show that PVMo undergoes fast multielectron transfer with intermediate Cu/RSH complexes, while PXW11 show far slower and limited electron transfer ability with these Cu/RSH complexes. Limited electron transfer between Cu nodes and the encapsulated POM units not only hinders reactivity but also leads to MOF framework distortion and subsequent decomposition induced by the reduction of Cu(ii) to Cu(i) sites in the framework.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


