基质佐剂
Q1 Pharmacology, Toxicology and Pharmaceutics
引用次数: 19
摘要
碳水化合物佐剂是安全且具有生物相容性的化合物,可作为持续递送系统和正在进行的体液和细胞免疫反应的兴奋剂,特别适用于开发针对细胞内病原体的疫苗,其中明矾是无用的。新佐剂的开发是困难和昂贵的,然而,在过去的两年中,七种新的碳水化合物佐剂已经获得专利,还有12种含有碳水化合物佐剂的疫苗正在进行临床试验,以及许多关于其作用机制和安全性的出版物。对现有佐剂进行改进和开发新型佐剂还需要进一步的研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
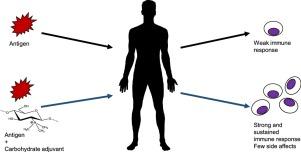
Carbohydrate-based adjuvants
Carbohydrate adjuvants are safe and biocompatible compounds usable as sustained delivery systems and stimulants of ongoing humoral and cellular immune responses, being especially suitable for the development of vaccines against intracellular pathogens where alum is useless. The development of new adjuvants is difficult and expensive, however, in the last two years, seven new carbohydrate-based adjuvants have been patented, also there are twelve ongoing clinical trials of vaccines that contain carbohydrate-based adjuvants, as well as numerous publications on their mechanism of action and safety. More research is necessary to improve the existent adjuvants and develop innovative ones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Drug Discovery Today: Technologies
Pharmacology, Toxicology and Pharmaceutics-Drug Discovery
自引率
0.00%
发文量
0
期刊介绍:
Discovery Today: Technologies compares different technological tools and techniques used from the discovery of new drug targets through to the launch of new medicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


