HDAC2将泛素化与滑膜肉瘤的肿瘤抑制联系起来。
IF 1.9
Q3 ONCOLOGY
引用次数: 5
摘要
组蛋白去乙酰化酶2 (HDAC2)在转录调控中的功能及其在肿瘤发生中的作用已经得到了很好的证实。在这里,我们讨论了通过小鼠双分钟2同源物(MDM2)泛素连接酶控制癌症相关蛋白稳定性的转录不依赖的HDAC2途径。在滑膜肉瘤中,HDAC2失活通过降解SS18-SSX驱动癌蛋白显示出显著的治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
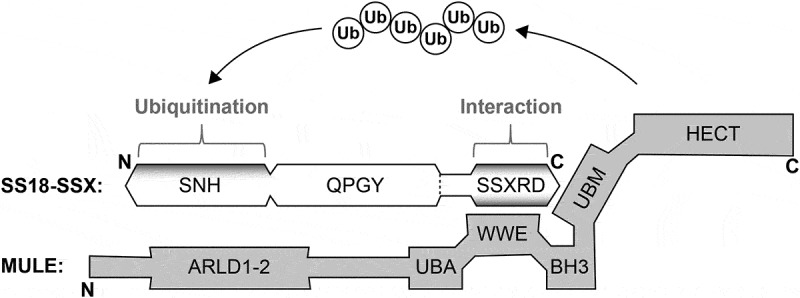
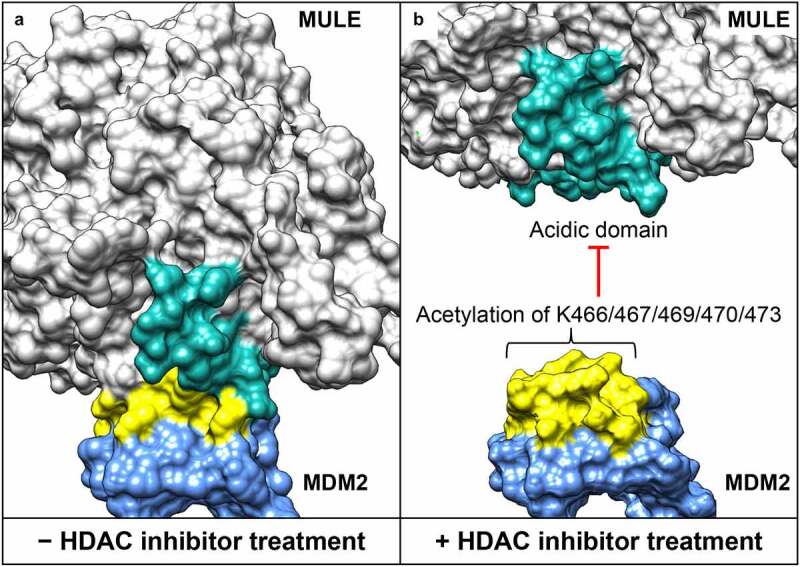

HDAC2 links ubiquitination to tumor suppression in synovial sarcoma.
The function of histone deacetylase 2 (HDAC2) in transcriptional regulation and its role in oncogenesis have been well established. Here we discuss a transcription-independent HDAC2 pathway controlling cancer-related protein stability via the mouse double minute 2 homolog (MDM2) ubiquitin ligase. In synovial sarcoma, HDAC2 inactivation demonstrates significant therapeutic effect by degradation of the SS18-SSX driver oncoprotein.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular and Cellular Oncology
Biochemistry, Genetics and Molecular Biology-Cancer Research
CiteScore
3.20
自引率
0.00%
发文量
18
期刊介绍:
For a long time, solid neoplasms have been viewed as relatively homogeneous entities composed for the most part of malignant cells. It is now clear that tumors are highly heterogeneous structures that evolve in the context of intimate interactions between cancer cells and endothelial, stromal as well as immune cells. During the past few years, experimental and clinical oncologists have witnessed several conceptual transitions of this type. Molecular and Cellular Oncology (MCO) emerges within this conceptual framework as a high-profile forum for the publication of fundamental, translational and clinical research on cancer. The scope of MCO is broad. Submissions dealing with all aspects of oncogenesis, tumor progression and response to therapy will be welcome, irrespective of whether they focus on solid or hematological neoplasms. MCO has gathered leading scientists with expertise in multiple areas of cancer research and other fields of investigation to constitute a large, interdisciplinary, Editorial Board that will ensure the quality of articles accepted for publication. MCO will publish Original Research Articles, Brief Reports, Reviews, Short Reviews, Commentaries, Author Views (auto-commentaries) and Meeting Reports dealing with all aspects of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


