铜催化2-羰基吲哚的1,4-羧酸酯脱芳重排†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01647h
引用次数: 0
摘要
在铜催化剂的存在下,2-羰基吲哚与芳基重氮乙酸酯进行了一种新的脱芳1,4-重排反应,该反应可能通过串联形成两性离子中间体进行,分子内环化和开环反应得到最终的重排产物,该重排产物含有一个具有两个新形成的C–C键的全碳四元中心。在这个序列中,吲哚的芳香性被破坏,与1,4-羧酸盐重排有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
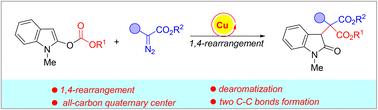
Copper-catalyzed dearomative 1,4-carboxylate rearrangement of 2-carbonateindoles†
A novel dearomative 1,4-rearrangement reaction of 2-carbonateindoles with aryl diazoacetates has been developed in the presence of a copper catalyst, which might proceed through a tandem formation of a zwitterionic intermediate, intramolecular cyclization and ring-opening reaction to give the final rearrangement products containing an all-carbon quaternary center with two newly formed C–C bonds. In this sequence, the disruption of the aromaticity of indole has been realized, associated with 1,4-carboxylate rearrangements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


