对映体选择性Ni催化炔烃连接的酮酰胺的同氢盐环化反应生成α-羟基-γ-内酰胺††
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00670k
引用次数: 0
摘要
报道了一种对映选择性镍催化炔烃连接酮酰胺的合成α-羟基γ-内酰胺的合成。以Ni(OTs)2·6H2O/(S,S)-Me Duphos为预催化剂,(EtO)2MeSiH为氢化物源,以32–84%的产率获得了一系列具有完全取代立体中心的对映体富集的γ-内酰胺,产率为87.5% : 12.5–97 : 3 er。还展示了合成效用,包括放大反应和产物衍生化。本研究提出了炔烃的区域选择性功能化,并为获得富含官能团的手性杂环提供了一种有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
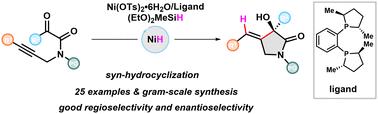
Enantioselective Ni-catalyzed syn-hydrometalative cyclization of alkyne-tethered ketoamides to α-hydroxy-γ-lactams†‡
An enantioselective Ni-catalyzed syn-hydrocyclization of alkyne-tethered ketoamides for the synthesis of α-hydroxy γ-lactams is reported. Using Ni(OTs)2·6H2O/(S,S)-Me-Duphos as a precatalyst and (EtO)2MeSiH as a hydride source, a broad range of enantioenriched γ-lactams with a fully substituted stereogenic center are obtained in 32–84% yields with 87.5 : 12.5–97 : 3 er. Synthetic utilities, including scale-up reaction and product derivatization, are also demonstrated. This research presents a regioselective functionalization of alkynes and provides an efficient strategy to access functional group-enriched chiral heterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


