Rh(iii)催化的C-H活化/ n -氨基甲酰亚砜酰脲环化反应用于三氟甲基取代(二氢)嘧啶吲哚酮的发散合成
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00803g
引用次数: 0
摘要
通过Rh(iii)催化的N-氨基甲酰基吲哚的C–H活化/环化与CF3酰亚磺酰亚磺酰基亚磺酰基化,实现了一种高效而直接的发散合成三氟甲基取代的(二氢)嘧啶吲哚酮的策略。级联反应可能通过C–H酰亚胺甲基化、互变异构化和分子内亲核加成序列进行。在氧化还原中性条件下,以良好至优异的产率构建了广泛的功能化嘧啶并吲哚酮衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
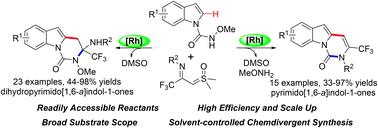
Rh(iii)-catalyzed C–H activation/annulation of N-carbamoylindoles with CF3-imidoyl sulfoxonium ylides for the divergent synthesis of trifluoromethyl-substituted (dihydro)pyrimidoindolones†
An efficient and straightforward strategy for the divergent synthesis of trifluoromethyl-substituted (dihydro)pyrimidoindolones via Rh(iii)-catalyzed C–H activation/annulation of N-carbamoylindoles with CF3-imidoyl sulfoxonium ylides has been achieved. The cascade reaction might proceed through a C–H imidoylmethylation, tautomerization and intramolecular nucleophilic addition sequence. A wide range of functionalized pyrimidoindolone derivatives were constructed in good to excellent yields under redox-neutral conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


