碱催化硅乙炔与酮的加成反应:生成受保护叔丙基醇的途径†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00579h
引用次数: 0
摘要
炔基硅烷与酮衍生物的碱催化加成使得能够形成各种硅烷基保护的炔丙醇。市售且廉价的双(三甲基甲硅烷基)酰胺钾(KHMDS)是一种有效的不含过渡金属的催化剂,并允许多种衍生物的功能化,包括药物和生物相关化合物。总的来说,所提出的系统补充了主要依赖化学计量过程或氟化物介导的反应的受保护的叔炔丙醇的经典途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
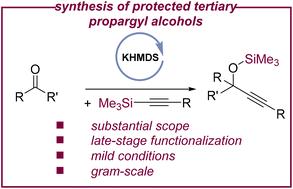
Base-catalyzed addition of silylacetylenes to ketones: a route to protected tertiary propargyl alcohols†
The base-catalyzed addition of alkynylsilanes to ketone derivatives enables the formation of various silyl-protected propargylic alcohols. Commercially available and inexpensive potassium bis(trimethylsilyl)amide (KHMDS) serves as an efficient transition metal-free catalyst and permits the functionalization of a variety of derivatives, including pharmaceuticals and biorelevant compounds. Overall, the presented system complements classical routes to protected tertiary propargylic alcohols that mainly rely on stoichiometric processes or fluoride-mediated reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


