n -丁二烯取代氧吲哚衍生物的高效合成
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2018-12-07
DOI:10.1039/c8qo00930a
引用次数: 1
摘要
报道了一种新的铑(iii)催化的酰胺羰基定向烯化反应,为合成有价值的多功能功能化N-(2E,4Z)-丁二烯取代的氧吲哚衍生物提供了一种有效的方法。吲哚类化合物作为一种新型的定向基团在本文中得到了很好的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
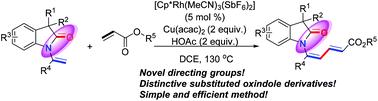
Efficient synthesis of N-butadiene substituted oxindole derivatives1
A novel rhodium(iii)-catalyzed amide carbonyl group directed alkenylation reaction between oxindoles and alkenes has been reported, which provides an efficient method for the synthesis of valuable and versatile functionalized N-(2E,4Z)-butadiene substituted oxindole derivatives. Oxindoles as novel directing groups have been well applied in this paper.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


