通过Ir催化的仲酰胺与酰氯的温和形式缩合反应一锅合成1,3-恶嗪-4-酮†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00092c
引用次数: 0
摘要
通过Ir催化的仲酰胺与己二酰氯和庚酰氯的一锅反应,分别合成了双环1,3-恶嗪-4-酮、2,3,6,7-四氢环戊二烯并[e]-1,3-恶津-4-酮和2,3,5,6,7,8-六氢-4H-苯并[e]-1,3-恶嗪-4-酮类。该方法具有产率高、底物范围广、反应条件温和、可扩展性强等特点。该反应还显示出与乙酰氯和苯乙酰氯的良好相容性,以获得单环1,3-恶嗪-4-酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
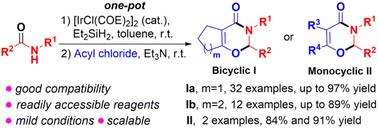
One-pot synthesis of 1,3-oxazin-4-ones through an Ir-catalyzed mild formal condensation reaction of secondary amides with acyl chlorides†
A facile and efficient synthesis of bicyclic 1,3-oxazin-4-ones, 2,3,6,7-tetrahydrocyclopenta[e]-1,3-oxazin-4-ones and 2,3,5,6,7,8-hexahydro-4H-benzo[e]-1,3-oxazin-4-ones, has been achieved via an Ir-catalyzed one-pot reaction of secondary amides with adipoyl chloride and pimeloyl chloride, respectively. This method features good yields, broad substrate scope, mild reaction conditions, and scalability. This reaction also shows good compatibility with acetyl chloride and phenylacetyl chloride to access monocyclic 1,3-oxazin-4-ones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


