钯催化的烯醇的级联环化/分子内氧化还原中继Heck芳基化:从2-(羟基烯基)磺酰苯胺获得四氢-β-卡宾†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00620d
引用次数: 0
摘要
提出了一种钯催化的级联反应,将钯催化的炔烃环化和烯醇的分子内氧化还原中继Heck芳基化相结合,由2-(羟基烯基)磺酰胺合成四氢-β-碳啉和其他多环吲哚。这种方法能够在一次操作中构建两个环并安装一个远程羰基,为进一步的精细化提供了广泛的合成用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
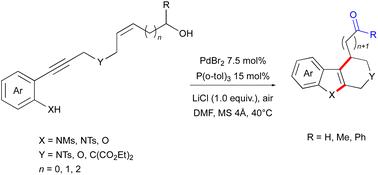
Palladium-catalyzed cascade cyclization/intramolecular redox-relay Heck arylation of alkenols: access to tetrahydro-β-carbolines from 2-(hydroxyalkenynyl)sulfonanilides†
A palladium-catalyzed cascade reaction for the synthesis of tetrahydro-β-carbolines and other polycyclic indoles from 2-(hydroxyenyl)sulfonanilides by combining the Pd(ii)-catalyzed cyclization of alkynes and intramolecular redox-relay Heck arylation of alkenols is presented. This method enables the construction of two rings and installs a remote carbonyl group in a single operation, offering broad synthetic utility for further elaborations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


