铑催化烯烃通过羰基化C–H活化对聚(杂)环烷基芳烯酮的氨基酰化†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01777f
引用次数: 0
摘要
用CO发达的羰基化合成各种有用的复杂分子是非常有效和有吸引力的。本文开发了在CO/O2的环境压力下,通过N–H键和C–H键的双重断裂,铑催化烯烃与侧酰基苯胺的模块化羰基化氨基苯甲酰化反应。以中高产率获得了各种聚(杂)环烷基芳基酮。还探讨了产品的逻辑综合转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
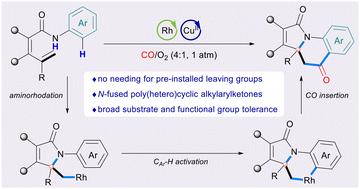
Rhodium-catalyzed aminoacylation of alkenes via carbonylative C–H activation toward poly(hetero)cyclic alkylarylketones†
The well-developed carbonylative synthesis of varied and useful complex molecules with CO is very efficient and attractive. Herein, rhodium-catalyzed modular carbonylative aminobenzoylation of alkenes with a pendant acylaniline enabled by dual cleavage of N–H bonds and C–H bonds under ambient pressure of CO/O2 was developed. Various poly(hetero)cyclic alkylarylketones were obtained in moderate-to-high yields. The logical synthetic transformations of products were also explored.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


