对映选择性N-杂环卡宾催化烯醇ε-内酯的重排†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01721k
引用次数: 0
摘要
β-内酯是生物活性分子中常见的亚结构。在此,我们开发了一种对映选择性N-杂环卡宾(NHC)催化的烯醇ε-内酯重排反应,用于构建双环β-内酯。该反应对外烯醇和内烯醇ε-内酯都有效,分别产生具有两个或三个连续立体中心的相应双环β-内酯。该反应的特点是原料易得,原子经济性100%,条件温和,非对映体和对映体选择性高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
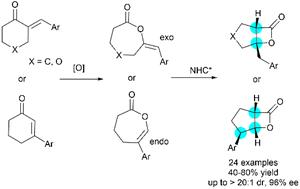
Enantioselective N-heterocyclic carbene-catalyzed rearrangement of enol ε-lactones†
β-Lactones are common substructures in bioactive molecules. Herein, we developed an enantioselective N-heterocyclic carbene (NHC)-catalyzed rearrangement of enol ε-lactones for the construction of bicyclic β-lactones. The reaction works well for both exo- and endo-enol ε-lactones, giving the corresponding bicyclic β-lactones with two or three contiguous stereocenters, respectively. The reaction features readily available starting materials, 100% atom economy, mild conditions, high diastereo- and enantioselectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


