Dina Dikovskaya, Albena T. Dinkova-Kostova
{"title":"用flm - fret测量单个细胞中Keap1-Nrf2蛋白复合物构象的变化","authors":"Dina Dikovskaya, Albena T. Dinkova-Kostova","doi":"10.1002/cptx.96","DOIUrl":null,"url":null,"abstract":"<p>The nuclear factor−erythroid 2 p45-related factor 2 (Nrf2)−mediated stress response is a major cellular defense mechanism against endogenous and exogenous oxidants, electrophiles, and pro-inflammatory agents. A number of Nrf2 inducers are being developed to therapeutically stimulate this pathway. Inducers are typically sensed by Kelch-like ECH-associated protein 1 (Keap1), a negative regulator and a binding partner of Nrf2. Modifications of Keap1 by oxidants or electrophiles, or its targeting by compounds that disrupt its interaction with Nrf2, alter the conformation of the Keap1-Nrf2 protein complex, which initiates the accumulation of Nrf2 required for mounting a stress response. To detect conformational changes in the Keap1-Nrf2 complex in live cells, we have developed a procedure based on Fluorescence Lifetime Imaging−Förster Resonance Energy Transfer (FLIM-FRET). The procedure includes a FLIM time course in cells expressing fluorescently-tagged Nrf2 and Keap1, followed by an extended analysis pipeline that quantifies changes in fluorescence lifetime of labeled Nrf2. The analysis visualizes and removes intensity-dependent bias in fluorescence lifetime measured with the Time-Correlated Single Photon Counting (TCSPC) approach, thereby improving the accuracy of quantification. The throughput is increased by the whole-experiment analysis within the newly developed FLIM dataset tool (FLIMDAST) and by the time-lapse FLIM described here. This pipeline is also suitable for applications beyond the Nrf2 field that assess small changes in fluorescence lifetime of objects with variable fluorescence intensities measured using TCSPC-based FLIM. © 2020 The Authors.</p><p><b>Basic Protocol 1</b>: Lipofectamine 2000 transfection</p><p><b>Alternate Protocol 1</b>: Calcium phosphate transfection</p><p><b>Basic Protocol 2</b>: Time course with individual FLIM</p><p><b>Alternate Protocol 2</b>: Time course with time-lapse FLIM</p><p><b>Support Protocol</b>: Measuring Instrument Response Function (IRF)</p><p><b>Basic Protocol 3</b>: Data analysis in SPCImage</p><p><b>Basic Protocol 4</b>: Data processing in ImageJ/FIJI</p><p><b>Basic Protocol 5</b>: Experiment analysis in FLIMDAST</p>","PeriodicalId":72743,"journal":{"name":"Current protocols in toxicology","volume":"85 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-08-12","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cptx.96","citationCount":"2","resultStr":"{\"title\":\"Measuring Changes in Keap1-Nrf2 Protein Complex Conformation in Individual Cells by FLIM-FRET\",\"authors\":\"Dina Dikovskaya, Albena T. Dinkova-Kostova\",\"doi\":\"10.1002/cptx.96\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The nuclear factor−erythroid 2 p45-related factor 2 (Nrf2)−mediated stress response is a major cellular defense mechanism against endogenous and exogenous oxidants, electrophiles, and pro-inflammatory agents. A number of Nrf2 inducers are being developed to therapeutically stimulate this pathway. Inducers are typically sensed by Kelch-like ECH-associated protein 1 (Keap1), a negative regulator and a binding partner of Nrf2. Modifications of Keap1 by oxidants or electrophiles, or its targeting by compounds that disrupt its interaction with Nrf2, alter the conformation of the Keap1-Nrf2 protein complex, which initiates the accumulation of Nrf2 required for mounting a stress response. To detect conformational changes in the Keap1-Nrf2 complex in live cells, we have developed a procedure based on Fluorescence Lifetime Imaging−Förster Resonance Energy Transfer (FLIM-FRET). The procedure includes a FLIM time course in cells expressing fluorescently-tagged Nrf2 and Keap1, followed by an extended analysis pipeline that quantifies changes in fluorescence lifetime of labeled Nrf2. The analysis visualizes and removes intensity-dependent bias in fluorescence lifetime measured with the Time-Correlated Single Photon Counting (TCSPC) approach, thereby improving the accuracy of quantification. The throughput is increased by the whole-experiment analysis within the newly developed FLIM dataset tool (FLIMDAST) and by the time-lapse FLIM described here. This pipeline is also suitable for applications beyond the Nrf2 field that assess small changes in fluorescence lifetime of objects with variable fluorescence intensities measured using TCSPC-based FLIM. © 2020 The Authors.</p><p><b>Basic Protocol 1</b>: Lipofectamine 2000 transfection</p><p><b>Alternate Protocol 1</b>: Calcium phosphate transfection</p><p><b>Basic Protocol 2</b>: Time course with individual FLIM</p><p><b>Alternate Protocol 2</b>: Time course with time-lapse FLIM</p><p><b>Support Protocol</b>: Measuring Instrument Response Function (IRF)</p><p><b>Basic Protocol 3</b>: Data analysis in SPCImage</p><p><b>Basic Protocol 4</b>: Data processing in ImageJ/FIJI</p><p><b>Basic Protocol 5</b>: Experiment analysis in FLIMDAST</p>\",\"PeriodicalId\":72743,\"journal\":{\"name\":\"Current protocols in toxicology\",\"volume\":\"85 1\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2020-08-12\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://sci-hub-pdf.com/10.1002/cptx.96\",\"citationCount\":\"2\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols in toxicology\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cptx.96\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in toxicology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cptx.96","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 2
Measuring Changes in Keap1-Nrf2 Protein Complex Conformation in Individual Cells by FLIM-FRET
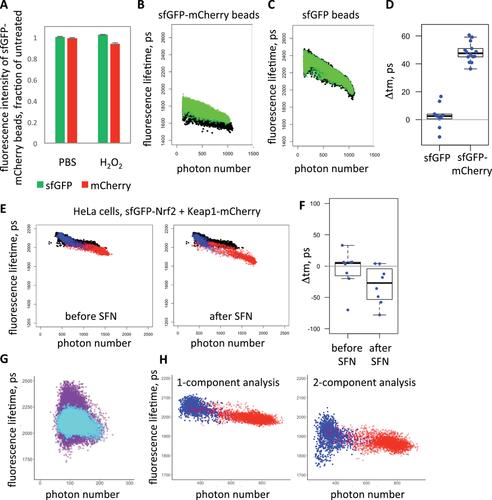
The nuclear factor−erythroid 2 p45-related factor 2 (Nrf2)−mediated stress response is a major cellular defense mechanism against endogenous and exogenous oxidants, electrophiles, and pro-inflammatory agents. A number of Nrf2 inducers are being developed to therapeutically stimulate this pathway. Inducers are typically sensed by Kelch-like ECH-associated protein 1 (Keap1), a negative regulator and a binding partner of Nrf2. Modifications of Keap1 by oxidants or electrophiles, or its targeting by compounds that disrupt its interaction with Nrf2, alter the conformation of the Keap1-Nrf2 protein complex, which initiates the accumulation of Nrf2 required for mounting a stress response. To detect conformational changes in the Keap1-Nrf2 complex in live cells, we have developed a procedure based on Fluorescence Lifetime Imaging−Förster Resonance Energy Transfer (FLIM-FRET). The procedure includes a FLIM time course in cells expressing fluorescently-tagged Nrf2 and Keap1, followed by an extended analysis pipeline that quantifies changes in fluorescence lifetime of labeled Nrf2. The analysis visualizes and removes intensity-dependent bias in fluorescence lifetime measured with the Time-Correlated Single Photon Counting (TCSPC) approach, thereby improving the accuracy of quantification. The throughput is increased by the whole-experiment analysis within the newly developed FLIM dataset tool (FLIMDAST) and by the time-lapse FLIM described here. This pipeline is also suitable for applications beyond the Nrf2 field that assess small changes in fluorescence lifetime of objects with variable fluorescence intensities measured using TCSPC-based FLIM. © 2020 The Authors.
Basic Protocol 1: Lipofectamine 2000 transfection
Alternate Protocol 1: Calcium phosphate transfection
Basic Protocol 2: Time course with individual FLIM
Alternate Protocol 2: Time course with time-lapse FLIM
Support Protocol: Measuring Instrument Response Function (IRF)
Basic Protocol 3: Data analysis in SPCImage
Basic Protocol 4: Data processing in ImageJ/FIJI
Basic Protocol 5: Experiment analysis in FLIMDAST

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


