Emelissa J. Mendoza, Kathy Manguiat, Heidi Wood, Michael Drebot
下载PDF
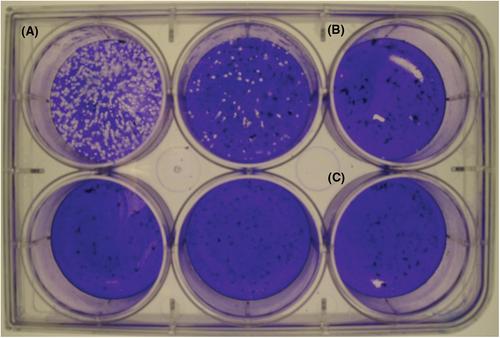
{"title":"传染性SARS-CoV-2定量的两种详细空斑测定方案","authors":"Emelissa J. Mendoza, Kathy Manguiat, Heidi Wood, Michael Drebot","doi":"10.1002/cpmc.105","DOIUrl":null,"url":null,"abstract":"<p>Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been identified as the causal agent of COronaVIrus Disease-19 (COVID-19), an atypical pneumonia-like syndrome that emerged in December 2019. While SARS-CoV-2 titers can be measured by detection of viral nucleic acid, this method is unable to quantitate infectious virions. Measurement of infectious SARS-CoV-2 can be achieved by tissue culture infectious dose−50 (TCID<sub>50</sub>), which detects the presence or absence of cytopathic effect in cells infected with serial dilutions of a virus specimen. However, this method only provides a qualitative infectious virus titer. Plaque assays are a quantitative method of measuring infectious SARS-CoV-2 by quantifying the plaques formed in cell culture upon infection with serial dilutions of a virus specimen. As such, plaque assays remain the gold standard in quantifying concentrations of replication-competent lytic virions. Here, we describe two detailed plaque assay protocols to quantify infectious SARS-CoV-2 using different overlay and staining methods. Both methods have several advantages and disadvantages, which can be considered when choosing the procedure best suited for each laboratory. These assays can be used for several research purposes, including titration of virus stocks produced from infected cell supernatant and, with further optimization, quantification of SARS-CoV-2 in specimens collected from infected animals. © 2019 The Authors.</p><p><b>Basic Protocol</b>: SARS-CoV-2 plaque assay using a solid double overlay method</p><p><b>Alternate Protocol</b>: SARS-CoV-2 plaque assay using a liquid overlay and fixation-staining method</p>","PeriodicalId":39967,"journal":{"name":"Current Protocols in Microbiology","volume":"57 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-05-31","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpmc.105","citationCount":"164","resultStr":"{\"title\":\"Two Detailed Plaque Assay Protocols for the Quantification of Infectious SARS-CoV-2\",\"authors\":\"Emelissa J. Mendoza, Kathy Manguiat, Heidi Wood, Michael Drebot\",\"doi\":\"10.1002/cpmc.105\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been identified as the causal agent of COronaVIrus Disease-19 (COVID-19), an atypical pneumonia-like syndrome that emerged in December 2019. While SARS-CoV-2 titers can be measured by detection of viral nucleic acid, this method is unable to quantitate infectious virions. Measurement of infectious SARS-CoV-2 can be achieved by tissue culture infectious dose−50 (TCID<sub>50</sub>), which detects the presence or absence of cytopathic effect in cells infected with serial dilutions of a virus specimen. However, this method only provides a qualitative infectious virus titer. Plaque assays are a quantitative method of measuring infectious SARS-CoV-2 by quantifying the plaques formed in cell culture upon infection with serial dilutions of a virus specimen. As such, plaque assays remain the gold standard in quantifying concentrations of replication-competent lytic virions. Here, we describe two detailed plaque assay protocols to quantify infectious SARS-CoV-2 using different overlay and staining methods. Both methods have several advantages and disadvantages, which can be considered when choosing the procedure best suited for each laboratory. These assays can be used for several research purposes, including titration of virus stocks produced from infected cell supernatant and, with further optimization, quantification of SARS-CoV-2 in specimens collected from infected animals. © 2019 The Authors.</p><p><b>Basic Protocol</b>: SARS-CoV-2 plaque assay using a solid double overlay method</p><p><b>Alternate Protocol</b>: SARS-CoV-2 plaque assay using a liquid overlay and fixation-staining method</p>\",\"PeriodicalId\":39967,\"journal\":{\"name\":\"Current Protocols in Microbiology\",\"volume\":\"57 1\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2020-05-31\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://sci-hub-pdf.com/10.1002/cpmc.105\",\"citationCount\":\"164\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current Protocols in Microbiology\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cpmc.105\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols in Microbiology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpmc.105","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 164
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


