通过Pechmann缩合/Mitsunobu反应/Claisen重排/烯烃交叉复分解序列组装戊烯基新黄酮体系。
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 2
摘要
摘要:以3-甲氧基苯乙酮为原料,经Mitsunobu反应/Claisen重排反应/烯烃交叉复分解反应,以5%的收率合成了戊烯基新黄酮。结果表明,该序列与Pechmann缩合反应相容,Pechmann缩合反应被证明是一种可靠且经济的α-吡咯酮核心组装方法。该结果为从苯酚和苯乙酮衍生物开始的戊烯基新黄酮体系的一般方法打开了大门。图形抽象:本文章由计算机程序翻译,如有差异,请以英文原文为准。
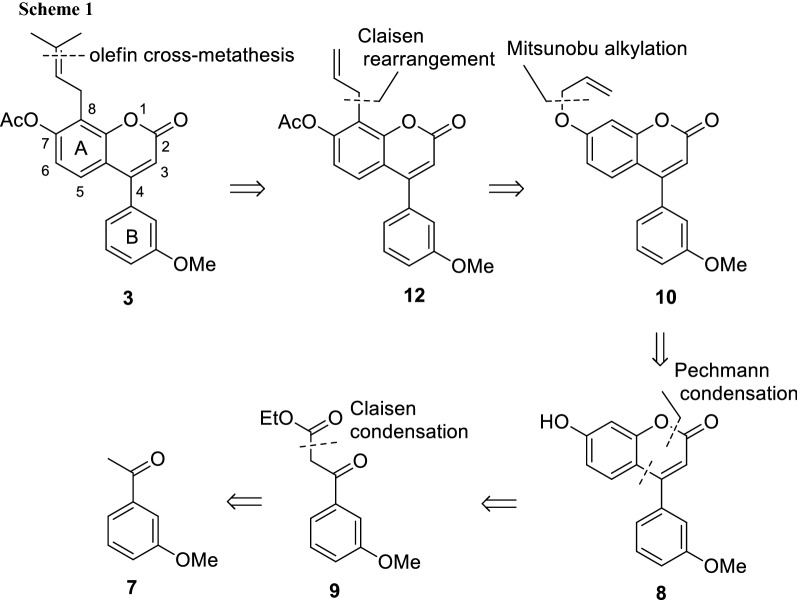
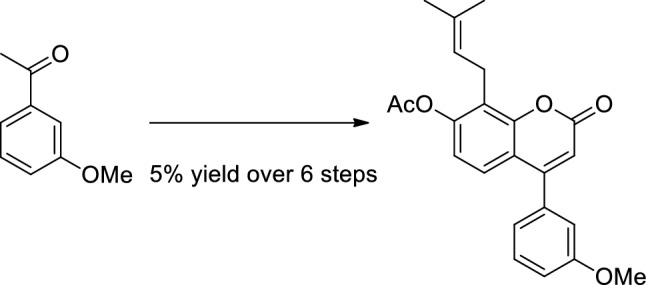
Assembling the prenylneoflavone system through a Pechmann condensation/Mitsunobu reaction/Claisen rearrangement/olefin cross-metathesis sequence.
Abstract: The multistep synthesis of a prenylneoflavone through a sequence of the Mitsunobu reaction/Claisen rearrangement/olefin cross-metathesis reaction has been accomplished in 5% yield over six steps starting from commercially available 3-methoxyacetophenone. The sequence is shown to be compatible with a Pechmann condensation which proved to be a robust and cost-effective method for the assembling of the α-pyrone core. The results open doors to a general approach to the prenylneoflavone system starting from phenol and acetophenone derivatives.
Graphic abstract:
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Monatshefte Fur Chemie
化学-化学综合
CiteScore
3.70
自引率
5.60%
发文量
116
审稿时长
3.3 months
期刊介绍:
"Monatshefte für Chemie/Chemical Monthly" was originally conceived as an Austrian journal of chemistry. It has evolved into an international journal covering all branches of chemistry. Featuring the most recent advances in research in analytical chemistry, biochemistry, inorganic, medicinal, organic, physical, structural, and theoretical chemistry, Chemical Monthly publishes refereed original papers and a section entitled "Short Communications". Reviews, symposia in print, and issues devoted to special fields will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


