组蛋白去乙酰化酶抑制剂:MEK抑制剂治疗葡萄膜黑色素瘤的有希望的合作伙伴?
IF 0.7
Q4 ONCOLOGY
引用次数: 3
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
Histone deacetylase inhibitors: a promising partner for MEK inhibitors in uveal melanoma?
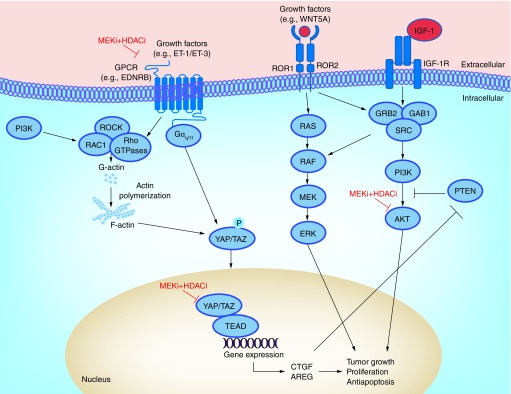
Uveal melanoma is a highly aggressive tumor derived from the melanocytes of the eye. More than 90% of uveal melanomas harbor activating mutations in the small G-proteins GNAQ/GNA11 and have constitutive activity in the MAPK pathway [1]. In uveal melanoma, GNAQ/GNA11 activates phospholipase C β, which cleaves phosphatidylinositol-4,5-biphosphate to diacyl glycerol and inositol triphosphate. Both of these products activate protein kinase C, which in turn activates the MAPK pathway. Constitutive signaling in other signal transduction cascades including the PI3K/AKT/mTOR, WNT/β-catenin and the YAP-signaling pathways have also been reported. Although approximately 4% of patients with uveal melanoma show signs of disseminated disease at diagnosis, approximately 4%, half eventually succumb to metastases [2]. The major site for uveal melanoma metastasis is the liver. For many uveal melanoma patients, development of metastases occurs many years after the successful treatment of the primary tumor. Patients can be stratified into low versus high risk of metastasis development (class 1 or class 2 uveal melanoma) on the basis of a 15-gene expression signature [3]. Class 1 tumors show greater melanocyte differentiation. Class 1 tumors can be further subdivided into class 1a and 1b categories with a 5-year metastasis risk of 2 and 21%, respectively [4]. Class 2 tumors typically lose melanocyte morphology and express genes associated with the primitive neuroectoderm. A class 2 gene signature is associated with a 5-year risk of metastasis equivalent to 70–80% [4]. One of the major genetic drivers of a class 2 phenotype is loss or inactivating mutations in the H2A ubiquitin hydrolase BAP1 [5]. Knockdown of BAP1 in uveal melanoma cell lines causes dedifferentiation and the adoption of a phenotype that confers metastatic behavior. BAP1 is the catalytic subunit of the poly comb repressive deubiquitinase (PR-DUB) complex that deubiquitinates histone H2A, and thus plays a key role in histone modification [6]. Recent work on the role of BAP1 in a Xenopus laevis development model has implicated it in the regulation of the epigenetic switch required for lineage commitment [7]. In this model, BAP1 loss was associated with transcriptional silencing and a failure of H3K27ac to accumulate at the promoters of key genes involved in lineage commitment including Sox2, Foxd3 and Sox10 [7]. Acetylation of histone H3 at lysine 27 (H3K27) is found at active and poised enhancer regions of genes. These data suggest that BAP1 loss leads to repression of lineage-specific gene expression, dedifferentiating the uveal melanoma cells to a primitive, embryonic-like state that favors metastasis. Once established in the liver, uveal melanomas respond very poorly to therapy options currently available, including targeted therapies, immunotherapies and chemotherapies [8]. There has been some suggestion that the relatively low mutational burden of uveal melanoma compared with cutaneous melanoma – resulting in a lower expression of tumor neoantigens – may underlie the lack of efficacy seen in immunotherapy. To date, most work in the targeted therapy arena has centered upon the inhibition of kinases downstream of GNAQ/GNA11. The
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Melanoma Management
ONCOLOGY-
CiteScore
5.10
自引率
0.00%
发文量
4
审稿时长
13 weeks
期刊介绍:
Skin cancer is on the rise. According to the World Health Organization, 132,000 melanoma skin cancers occur globally each year. While early-stage melanoma is usually relatively easy to treat, once disease spreads prognosis worsens considerably. Therefore, research into combating advanced-stage melanoma is a high priority. New and emerging therapies, such as monoclonal antibodies, B-RAF and KIT inhibitors, antiangiogenic agents and novel chemotherapy approaches hold promise for prolonging survival, but the search for a cure is ongoing. Melanoma Management publishes high-quality peer-reviewed articles on all aspects of melanoma, from prevention to diagnosis and from treatment of early-stage disease to late-stage melanoma and metastasis. The journal presents the latest research findings in melanoma research and treatment, together with authoritative reviews, cutting-edge editorials and perspectives that highlight hot topics and controversy in the field. Independent drug evaluations assess newly approved medications and their role in clinical practice. Key topics covered include: Risk factors, prevention and sun safety education Diagnosis, staging and grading Surgical excision of melanoma lesions Sentinel lymph node biopsy Biological therapies, including immunotherapy and vaccination Novel chemotherapy options Treatment of metastasis Prevention of recurrence Patient care and quality of life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


