Laise Rosado-Souza, Laure C. David, Margit Drapal, Paul D. Fraser, Jörg Hofmann, Patrick A. W. Klemens, Frank Ludewig, H. Ekkehard Neuhaus, Toshihiro Obata, Laura Perez-Fons, Armin Schlereth, Uwe Sonnewald, Mark Stitt, Samuel C. Zeeman, Wolfgang Zierer, Alisdair R. Fernie
下载PDF
{"title":"木薯代谢组学与淀粉品质","authors":"Laise Rosado-Souza, Laure C. David, Margit Drapal, Paul D. Fraser, Jörg Hofmann, Patrick A. W. Klemens, Frank Ludewig, H. Ekkehard Neuhaus, Toshihiro Obata, Laura Perez-Fons, Armin Schlereth, Uwe Sonnewald, Mark Stitt, Samuel C. Zeeman, Wolfgang Zierer, Alisdair R. Fernie","doi":"10.1002/cppb.20102","DOIUrl":null,"url":null,"abstract":"<p>Cassava plays an important role as a staple food for more than 800 million people in the world due to its ability to maintain relatively high productivity even in nutrient-depleted soils. Even though cassava has been the focus of several breeding programs and has become a strong focus of research in the last few years, relatively little is currently known about its metabolism and metabolic composition in different tissues. In this article, the absolute content of sugars, organic acids, amino acids, phosphorylated intermediates, minerals, starch, carotenoids, chlorophylls, tocopherols, and total protein as well as starch quality is described based on multiple analytical techniques, with protocols specifically adjusted for material from different cassava tissues. Moreover, quantification of secondary metabolites relative to internal standards is presented using both non-targeted and targeted metabolomics approaches. The protocols have also been adjusted to apply to freeze-dried material in order to allow processing of field harvest samples that typically will require long-distance transport. © 2019 The Authors.</p><p><b>Basic Protocol 1</b>: Metabolic profiling by gas chromatography–mass spectrometry (GC-MS)</p><p><b>Support Protocol 1</b>: Preparation of freeze-dried cassava material</p><p><b>Support Protocol 2</b>: Preparation of standard compound mixtures for absolute quantification of metabolites by GC-MS</p><p><b>Support Protocol 3</b>: Preparation of retention-time standard mixture</p><p><b>Basic Protocol 2</b>: Determination of organic acids and phosphorylated intermediates by ion chromatography–mass spectrometry (IC-MS)</p><p><b>Support Protocol 4</b>: Preparation of standards and recovery experimental procedure</p><p><b>Basic Protocol 3</b>: Determination of soluble sugars, starch, and free amino acids</p><p><b>Alternate Protocol</b>: Determination of soluble sugars and starch</p><p><b>Basic Protocol 4</b>: Determination of anions</p><p><b>Basic Protocol 5</b>: Determination of elements</p><p><b>Basic Protocol 6</b>: Determination of total protein</p><p><b>Basic Protocol 7</b>: Determination of non-targeted and targeted secondary metabolites</p><p><b>Basic Protocol 8</b>: Determination of carotenoids, chlorophylls, and tocopherol</p><p><b>Basic Protocol 9</b>: Determination of starch quality</p>","PeriodicalId":10932,"journal":{"name":"Current protocols in plant biology","volume":"4 4","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2019-12-13","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cppb.20102","citationCount":"13","resultStr":"{\"title\":\"Cassava Metabolomics and Starch Quality\",\"authors\":\"Laise Rosado-Souza, Laure C. David, Margit Drapal, Paul D. Fraser, Jörg Hofmann, Patrick A. W. Klemens, Frank Ludewig, H. Ekkehard Neuhaus, Toshihiro Obata, Laura Perez-Fons, Armin Schlereth, Uwe Sonnewald, Mark Stitt, Samuel C. Zeeman, Wolfgang Zierer, Alisdair R. Fernie\",\"doi\":\"10.1002/cppb.20102\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Cassava plays an important role as a staple food for more than 800 million people in the world due to its ability to maintain relatively high productivity even in nutrient-depleted soils. Even though cassava has been the focus of several breeding programs and has become a strong focus of research in the last few years, relatively little is currently known about its metabolism and metabolic composition in different tissues. In this article, the absolute content of sugars, organic acids, amino acids, phosphorylated intermediates, minerals, starch, carotenoids, chlorophylls, tocopherols, and total protein as well as starch quality is described based on multiple analytical techniques, with protocols specifically adjusted for material from different cassava tissues. Moreover, quantification of secondary metabolites relative to internal standards is presented using both non-targeted and targeted metabolomics approaches. The protocols have also been adjusted to apply to freeze-dried material in order to allow processing of field harvest samples that typically will require long-distance transport. © 2019 The Authors.</p><p><b>Basic Protocol 1</b>: Metabolic profiling by gas chromatography–mass spectrometry (GC-MS)</p><p><b>Support Protocol 1</b>: Preparation of freeze-dried cassava material</p><p><b>Support Protocol 2</b>: Preparation of standard compound mixtures for absolute quantification of metabolites by GC-MS</p><p><b>Support Protocol 3</b>: Preparation of retention-time standard mixture</p><p><b>Basic Protocol 2</b>: Determination of organic acids and phosphorylated intermediates by ion chromatography–mass spectrometry (IC-MS)</p><p><b>Support Protocol 4</b>: Preparation of standards and recovery experimental procedure</p><p><b>Basic Protocol 3</b>: Determination of soluble sugars, starch, and free amino acids</p><p><b>Alternate Protocol</b>: Determination of soluble sugars and starch</p><p><b>Basic Protocol 4</b>: Determination of anions</p><p><b>Basic Protocol 5</b>: Determination of elements</p><p><b>Basic Protocol 6</b>: Determination of total protein</p><p><b>Basic Protocol 7</b>: Determination of non-targeted and targeted secondary metabolites</p><p><b>Basic Protocol 8</b>: Determination of carotenoids, chlorophylls, and tocopherol</p><p><b>Basic Protocol 9</b>: Determination of starch quality</p>\",\"PeriodicalId\":10932,\"journal\":{\"name\":\"Current protocols in plant biology\",\"volume\":\"4 4\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2019-12-13\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://sci-hub-pdf.com/10.1002/cppb.20102\",\"citationCount\":\"13\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols in plant biology\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cppb.20102\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"Agricultural and Biological Sciences\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in plant biology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cppb.20102","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"Agricultural and Biological Sciences","Score":null,"Total":0}
引用次数: 13
引用
批量引用
Cassava Metabolomics and Starch Quality
Cassava plays an important role as a staple food for more than 800 million people in the world due to its ability to maintain relatively high productivity even in nutrient-depleted soils. Even though cassava has been the focus of several breeding programs and has become a strong focus of research in the last few years, relatively little is currently known about its metabolism and metabolic composition in different tissues. In this article, the absolute content of sugars, organic acids, amino acids, phosphorylated intermediates, minerals, starch, carotenoids, chlorophylls, tocopherols, and total protein as well as starch quality is described based on multiple analytical techniques, with protocols specifically adjusted for material from different cassava tissues. Moreover, quantification of secondary metabolites relative to internal standards is presented using both non-targeted and targeted metabolomics approaches. The protocols have also been adjusted to apply to freeze-dried material in order to allow processing of field harvest samples that typically will require long-distance transport. © 2019 The Authors.
Basic Protocol 1 : Metabolic profiling by gas chromatography–mass spectrometry (GC-MS)
Support Protocol 1 : Preparation of freeze-dried cassava material
Support Protocol 2 : Preparation of standard compound mixtures for absolute quantification of metabolites by GC-MS
Support Protocol 3 : Preparation of retention-time standard mixture
Basic Protocol 2 : Determination of organic acids and phosphorylated intermediates by ion chromatography–mass spectrometry (IC-MS)
Support Protocol 4 : Preparation of standards and recovery experimental procedure
Basic Protocol 3 : Determination of soluble sugars, starch, and free amino acids
Alternate Protocol : Determination of soluble sugars and starch
Basic Protocol 4 : Determination of anions
Basic Protocol 5 : Determination of elements
Basic Protocol 6 : Determination of total protein
Basic Protocol 7 : Determination of non-targeted and targeted secondary metabolites
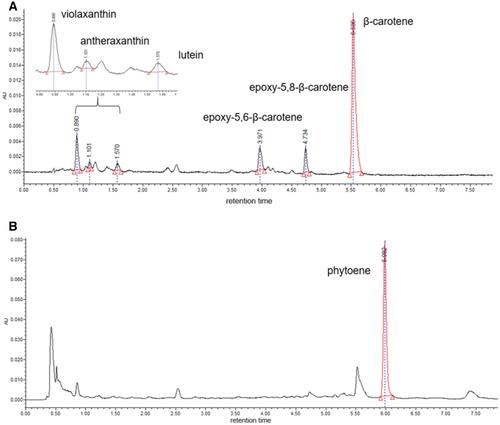
Basic Protocol 8 : Determination of carotenoids, chlorophylls, and tocopherol
Basic Protocol 9 : Determination of starch quality

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


