舌螺虫α- n -乙酰半乳糖胺酶转化红细胞的研究。
Q2 Biochemistry, Genetics and Molecular Biology
引用次数: 2
摘要
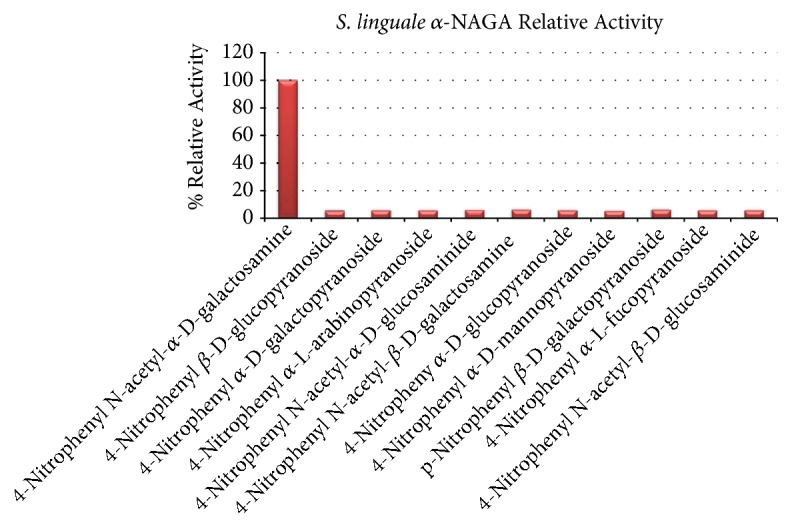
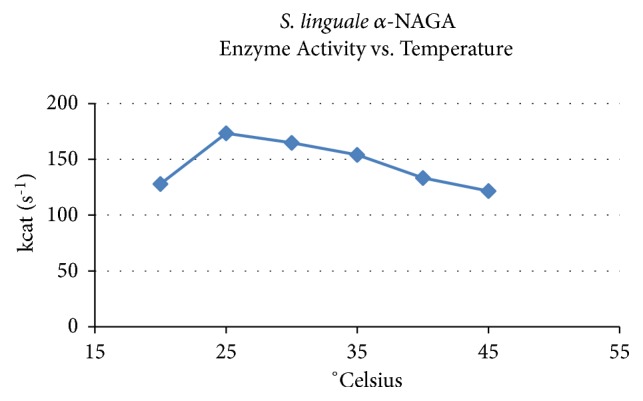
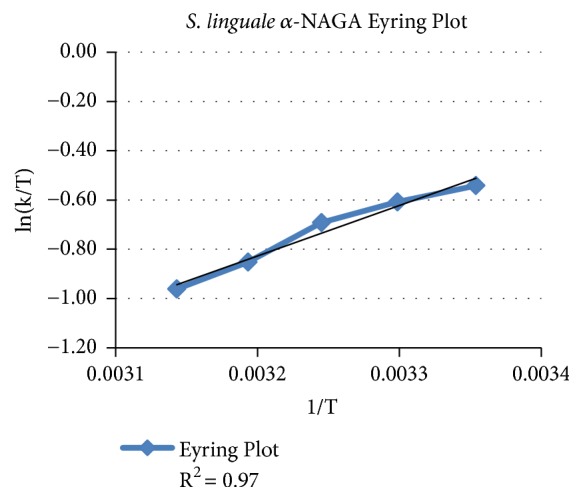
舌螺虫是一种自由生活的非致病性生物。像许多其他细菌一样,S. linguale产生与细胞相关的α- n -乙酰半乳糖胺酶。进行这项工作是为了阐明这项活动的性质。重组酶的产生、纯化和生化特性检测。纯化后的酶在溶液中为同二聚体,活性约50 kDa。在pH为7时催化α- n -乙酰半乳糖胺水解。计算KM为1.1 mM, kcat为173 s-1。所描述的酶属于GH109家族。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enzymatic Conversion of RBCs by α-N-Acetylgalactosaminidase from Spirosoma linguale.
Spirosoma linguale is a free-living nonpathogenic organism. Like many other bacteria, S. linguale produces a cell-associated α-N-acetylgalactosaminidase. This work was undertaken to elucidate the nature of this activity. The recombinant enzyme was produced, purified, and examined for biochemical attributes. The purified enzyme was ~50 kDa active as a homodimer in solution. It catalyzed hydrolysis of α-N-acetylgalactosamine at pH 7. Calculated KM was 1.1 mM with kcat of 173 s-1. The described enzyme belongs to the GH109 family.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Enzyme Research
Biochemistry, Genetics and Molecular Biology-Biochemistry
CiteScore
4.60
自引率
0.00%
发文量
0
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


