作为溶酶体 β-葡糖脑配体的改性 1,5-亚氨基-d-xylitols 的合成。
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
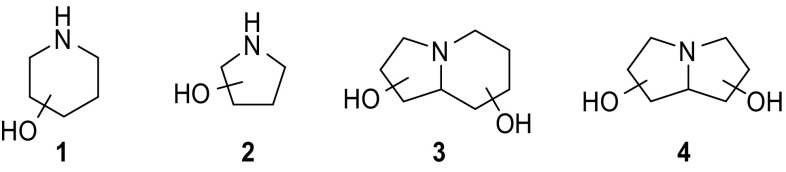
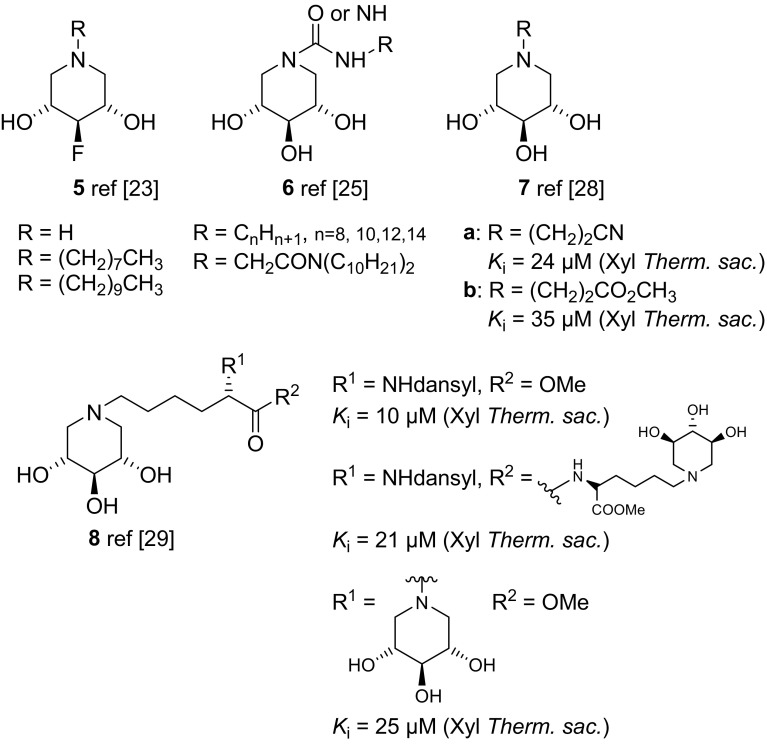
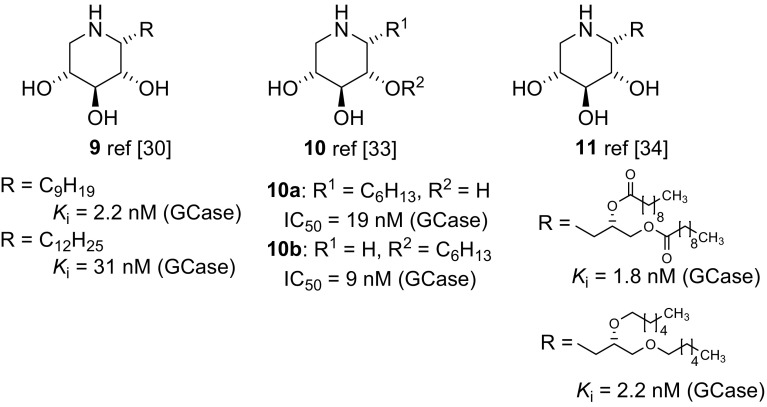
摘要:制备了涉及 C-1 位和/或环氮的不同取代模式的改性 1,5-二脱氧-1,5-亚氨基-d-木糖醇类似物,旨在将其作为制备亚氨基木糖醇基配体的前体以及阐明和调节人类溶酶体 β-葡糖脑苷脂的工具。用一系列糖苷水解酶对合成的拟糖体进行生物学评估后发现,这些取代模式具有极佳的β-葡萄糖苷酶选择性:本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis of modified 1,5-imino-d-xylitols as ligands for lysosomal β-glucocerebrosidase.
Abstract: Modified 1,5-dideoxy-1,5-imino-d-xylitol analogues with different substitution patterns involving position C-1 and/or the ring nitrogen were prepared, which were designed to serve as precursors for the preparation of iminoxylitol-based ligands and tools for the elucidation and modulation of human lysosomal β-glucocerebrosidase. Biological evaluation of the synthesized glycomimetics with a series of glycoside hydrolases revealed that these substitution patterns elicit excellent β-glucosidase selectivities.
Graphical abstract:
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Monatshefte Fur Chemie
化学-化学综合
CiteScore
3.70
自引率
5.60%
发文量
116
审稿时长
3.3 months
期刊介绍:
"Monatshefte für Chemie/Chemical Monthly" was originally conceived as an Austrian journal of chemistry. It has evolved into an international journal covering all branches of chemistry. Featuring the most recent advances in research in analytical chemistry, biochemistry, inorganic, medicinal, organic, physical, structural, and theoretical chemistry, Chemical Monthly publishes refereed original papers and a section entitled "Short Communications". Reviews, symposia in print, and issues devoted to special fields will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


