酮的有效直接膦化和烷基化以构建C–P和C–C键:获得α,α-二取代的γ-酮膦氧化物†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01749k
引用次数: 0
摘要
在无金属和无溶剂的条件下,描述了酸促进酮的二官能化,通过磷酸羟醛消除构建C–P和C–C键的第一个例子。级联的α-磷酸化和α-烷基化序列将酮直接转化为α,α-二取代的γ-酮氧化膦,从而为以水为唯一副产物以中等至优异的产率合成α,α二取代的α-酮氧化磷抗胆碱酯酶骨架提供了一种新的策略。详细的机理实验证实,反应通过酮的α-磷酸化后形成的TfOH诱导的碳阳离子中间体进行。本文章由计算机程序翻译,如有差异,请以英文原文为准。
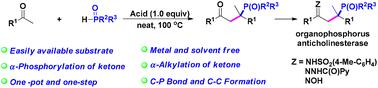
An efficient direct phosphinylation and alkylation of ketones to construct C–P and C–C bonds: access to α,α-disubstituted γ-ketophosphine oxides†
The first example of an acid-promoted difunctionalization of ketones to construct C–P and C–C bonds via a phospha-aldol-elimination is described under metal- and solvent-free conditions. The cascade α-phosphorylation and α-alkylation sequence directly converts ketones to α,α-disubstituted γ-ketone phosphine oxides, thereby providing a new strategy for the synthesis of the α,α-disubstituted γ-ketone phosphine oxide anticholinesterase skeleton in moderate to excellent yields with water as the only by-product. Detailed mechanistic experiments verified that the reaction proceeds via a TfOH-induced carbocationic intermediate formed after the α-phosphorylation of ketones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


