通过Matteson同系物/闭环复分解立体选择性合成五元和六元碳环†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00457k
引用次数: 0
摘要
Matteson同系物被发现是立体选择性合成多不饱和烷基硼酸酯的一种通用工具,这些酯是通过闭环复分解构建五元和六元碳环的优秀前体。Matteson反应的高度多样性允许制备高度取代的环状硼酸酯,其也适用于进一步的同源性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
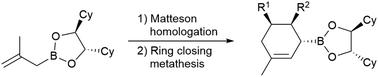
Stereoselective synthesis of five- and six-membered carbocycles via Matteson homologation/ring closing metathesis†
The Matteson homologation is found to be a versatile tool for the stereoselective synthesis of polyunsaturated alkyl boronic esters, which are excellent precursors for the construction of five- and six-membered carbocycles via ring-closing metathesis. The high diversity of the Matteson reaction allows for the preparation of highly substituted cyclic boronic esters, which are also suitable for further homologations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


