通过束缚反离子定向催化策略,对映选择性Au(i)催化2-炔基烯酮和萘酚之间的串联反应†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00415e
引用次数: 0
摘要
根据束缚反离子定向催化(TCDC)策略,使用具有膦-磷酸手性配体的金(i)催化剂研究了2-烷基烯酮与1-和2-萘酚的对映选择性串联环异构化/加成反应。反应在没有银添加剂的低催化剂负载量(0.2–1 mol%)下发生,萘酚同时作为O-和C-亲核试剂,产生具有高对映选择性的相应加成产物。DFT计算启发了这些过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
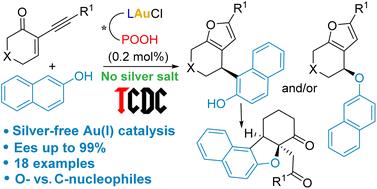
Enantioselective Au(i)-catalyzed tandem reactions between 2-alkynyl enones and naphthols by the tethered counterion-directed catalysis strategy†
Enantioselective tandem cycloisomerization/addition reactions of 2-alkynyl enones with 1- and 2-naphthols have been investigated using gold(i) catalysts featuring hybrid phosphine–phosphoric acid chiral ligands, according to the tethered counterion-directed catalysis (TCDC) strategy. The reactions occur at low catalyst loading (0.2–1 mol%) without silver additives, and the naphthols act as both O- and C-nucleophiles, leading to the corresponding addition products with high enantioselectivity. DFT calculations enlighten these processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


