尼鲁胺在转移性前列腺癌行睾丸切除术患者中的疗效和安全性:一项系统评价和荟萃分析。
IF 3.2
Q2 Pharmacology, Toxicology and Pharmaceutics
引用次数: 4
摘要
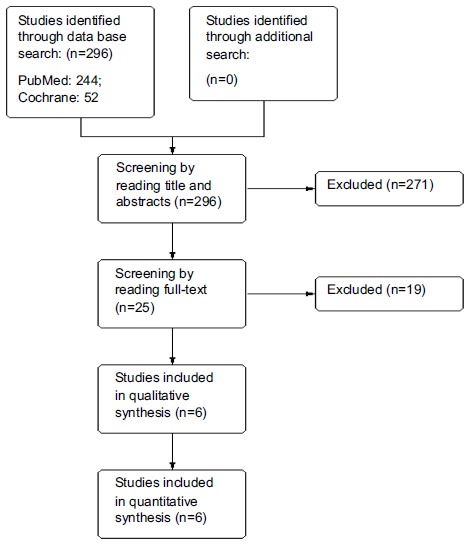
背景:前列腺癌是所有癌症死亡中的第六大死因,到2030年,这一负担预计将增加170万新病例和49.9万新死亡病例。我们的目的是评估尼鲁胺在转移性前列腺癌(mPCa)患者行睾丸切除术的疗效和安全性。方法:在Medline/PubMed和Cochrane图书馆进行综合检索。从纳入的研究和clinicaltrials.gov上的研究中寻找参考文献,没有语言和日期限制。我们只纳入随机对照试验,比较尼鲁胺在行睾丸切除术的转移性前列腺癌(mPCa)患者中的安全性和有效性。关注的结果是生存和药物反应和安全性。采用Cochrane偏倚风险工具评估纳入研究的质量。两位作者独立参与研究选择、数据提取和质量评估。两位审稿人之间的分歧通过咨询第三位审稿人来解决。结果:244项研究中有5项纳入荟萃分析,涉及1637名参与者。结论:有证据表明,与安慰剂组相比,接受尼鲁胺治疗的mPCa患者在无进展生存期、总生存反应率和临床获益方面均有显著改善。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficacy and Safety of Nilutamide in Patients with Metastatic Prostate Cancer who Underwent Orchiectomy: A Systematic Review and Metaanalysis.
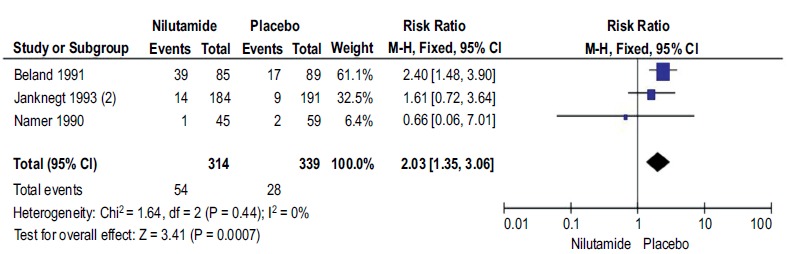
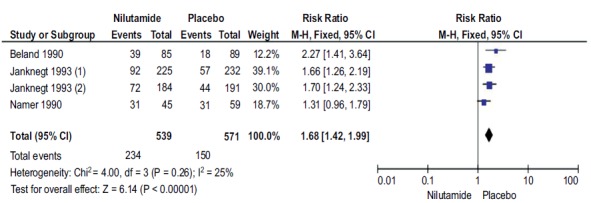
Background Prostate cancer is the sixth leading cause of death, among all cancer deaths By 2030, this burden is expected to increase with 1.7 million new cases and 499,000 new deaths. We aimed to evaluate the efficacy and safety of Nilutamide in metastatic prostate cancer (mPCa) patients who underwent orchiectomy. Methods A comprehensive search was conducted in the Medline/PubMed and Cochrane Library. References from included studies and studies from clinicaltrials.gov were explored without language and date restrictions. We included only randomized controlled trials, comparing the safety and efficacy of Nilutamide in Metastatic Prostate Cancer (mPCa) patients who underwent orchiectomy with placebo. The outcomes of concerns were survival and the response of drug and safety.. Quality of the included studies was assessed using the Cochrane Risk of Bias Tool. Two authors were independently involved in the study selection, data extraction and quality assessment. Disagreements between the two reviewers were resolved by consulting a third reviewer. Results A total of five out of 244 studies were included in meta-analysis involving1637 participants. Nilutamide group showed improved response rate (RR=1.77, 95%CI 1.46-2.14, p<0.00001), disease progression (RR=0.59, 95%CI 0.47-0.73, p<0.00001), complete response (RR=2.13, 95%CI 1.40-3.23, p=0.003) and clinical benefit (RR=1.23, 95%CI 1.13-1.34, p<0.00001) when compared to placebo; however, stable disease favored the control group (RR=0.80, 95%CI 0.68-0.94, p=0.007). In addition, patients on Nilutamide showed prolonged progression-free survival and overall survival. Nausea and vomiting were the most common adverse events reported in Nilutamide group. Conclusion Evidence suggests that patients with mPCa who underwent orchiectomy receiving Nilutamide showed significant improvement in progression-free survival and overall survival response rate and clinical benefits in comparison with the placebo group.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current clinical pharmacology
PHARMACOLOGY & PHARMACY-
CiteScore
3.60
自引率
0.00%
发文量
0
期刊介绍:
Current Clinical Pharmacology publishes frontier reviews on all the latest advances in clinical pharmacology. The journal"s aim is to publish the highest quality review articles in the field. Topics covered include: pharmacokinetics; therapeutic trials; adverse drug reactions; drug interactions; drug metabolism; pharmacoepidemiology; and drug development. The journal is essential reading for all researchers in clinical pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


