N-氟烷基三唑的Aza-Wolff重排反应生成烯酮胺†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00618b
引用次数: 0
摘要
N-氟烷基化的1,2,3-三唑经过微波加热辅助开环、氮分子消除和伴随的基团重排,形成可分离的N-氟烷基烯酮胺。这种无试剂工艺的特点是范围广、效率高,为制备未开发的N-氟烷基化合物提供了一条新途径。反应机理通过机理和计算研究相结合的方法进行了研究。烯酮胺与炔烃或烯烃的[2+2]环加成分别得到新的环丁烯亚胺和环丁亚胺。将氧、硫和氮亲核试剂添加到烯酮胺中,得到新的N-氟烷基酰亚胺、硫代酰亚胺和脒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
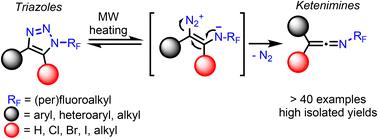
Aza-Wolff rearrangement of N-fluoroalkyl triazoles to ketenimines†
N-Fluoroalkylated 1,2,3-triazoles underwent a microwave-heating-assisted ring opening, nitrogen molecule elimination and concomitant group rearrangement to form isolable N-fluoroalkylketenimines. This reagent-free process is characterized by a wide scope and high efficiency and provides a new route to unexplored N-fluoroalkyl compounds. The reaction mechanism was investigated by a combination of mechanistic and computational studies. [2 + 2] cycloaddition of ketenimines with alkynes or alkenes afforded novel cyclobutenimines and cyclobutanimines, respectively. Addition of oxygen-, sulfur- and nitrogen nucleophiles to ketenimines gave new N-fluoroalkyl imidates, thioimidates and amidines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


