羧酸辅助环烯基和烷基溴化物之间空间要求很高的还原性交叉偶联†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00155e
引用次数: 0
摘要
开发了2-溴环烯基羧酸和烷基溴化物之间的镍催化还原交叉偶联反应,以高达92%的中等至优异产率提供全碳四取代环烯烃。机理研究表明,羧酸基团在这种空间要求很高的偶联反应中起着重要的辅助作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
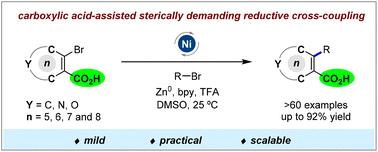
Carboxylic acid-assisted sterically demanding reductive cross-coupling between cycloalkenyl and alkyl bromides†
A nickel-catalysed reductive cross-coupling reaction between 2-bromo cycloalkenyl carboxylic acids and alkyl bromides has been developed, affording all-carbon tetrasubstituted cycloalkenes in moderate to excellent yields up to 92%. Mechanistic studies indicated that the carboxylic acid group plays an important assisting role in this sterically demanding coupling reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


