一类新的邻碳硼基联苯氧肟衍生物的合成及生物学评价。
Q1 Chemistry
引用次数: 2
摘要
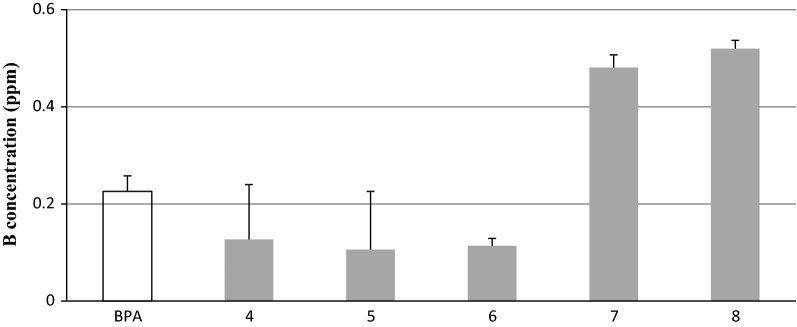
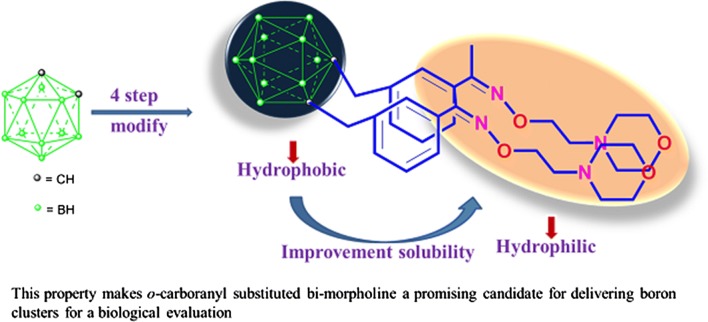
通过三步反应合成了(Z,Z′)-1,1′-(4-邻甲氧基苯基二甲基)-双(2-甲氧基苯基-1-肟)中间体3,最后以碱处理,得到了一系列新的邻甲氧基联苯二肟衍生物(4-8)。化合物7和8具有高溶解度,体外研究结果显示,与l -硼苯丙氨酸相比,化合物7和8在HeLa细胞中具有较高的积累水平,具有更高的细胞毒性和硼吸收率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and biological evaluation of a new series of ortho-carboranyl biphenyloxime derivatives.
(Z,Z')-1,1'-(4-ortho-Caboranyldimethyl)-bis(2-methoxyphenylethan-1-oxime) intermediate 3 was synthesized by a three-step reaction with a final treatment with base to give a new series of ortho-carboranyl biphenyloxime derivatives (4-8). Compounds 7 and 8 showed high solubility and the in vitro study results revealed high levels of accumulation in HeLa cells with higher cytotoxicity and boron uptake compared to L-boronphenylalanine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry Central Journal
化学-化学综合
CiteScore
4.40
自引率
0.00%
发文量
0
审稿时长
3.5 months
期刊介绍:
BMC Chemistry is an open access, peer reviewed journal that considers all articles in the broad field of chemistry, including research on fundamental concepts, new developments and the application of chemical sciences to broad range of research fields, industry, and other disciplines. It provides an inclusive platform for the dissemination and discussion of chemistry to aid the advancement of all areas of research.
Sections:
-Analytical Chemistry
-Organic Chemistry
-Environmental and Energy Chemistry
-Agricultural and Food Chemistry
-Inorganic Chemistry
-Medicinal Chemistry
-Physical Chemistry
-Materials and Macromolecular Chemistry
-Green and Sustainable Chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


