α-烷氧基甲基三苯基碘化磷的简易合成:PPh3/I2的新应用。
Q1 Chemistry
引用次数: 6
摘要
采用PPh3/I2在室温下一锅法合成了α-烷氧甲基碘化磷。发现反应条件一般,可合成多种结构变异的烷氧甲基碘化磷,收率高(70-91%)。这些新的功能化磷盐进一步用于乙烯醚的立体选择性合成以及醛的碳同源化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
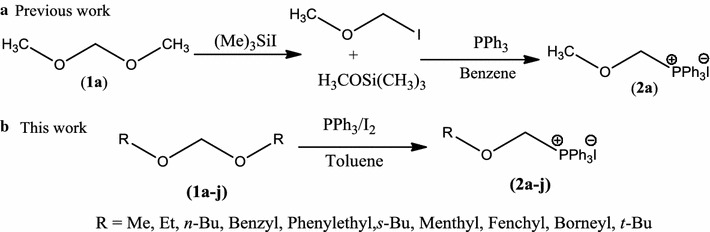
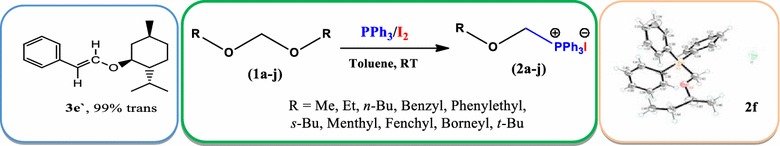
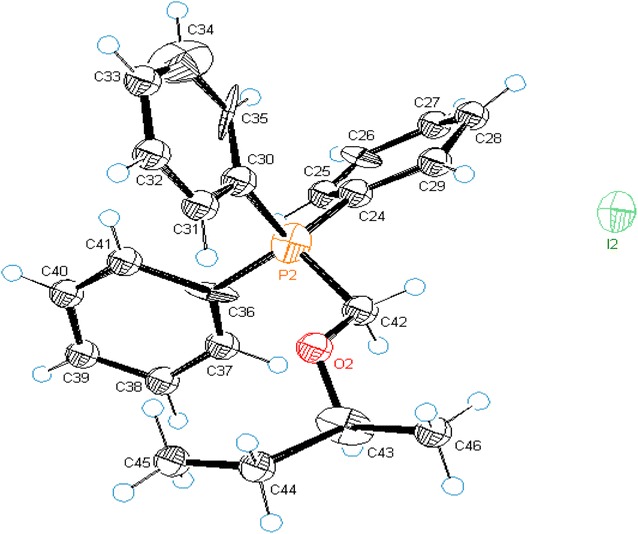
Facile synthesis of α-alkoxymethyltriphenylphosphonium iodides: new application of PPh3/I2.
An efficient one pot method for the synthesis of α-alkoxymethylphosphonium iodides is developed by using PPh3/I2 combination at room temperature. Reaction conditions are found general to synthesize wide range of structurally variant alkoxymethylphosphonium iodides in high yield (70-91%). These new functionalized phosphonium salts are further used in stereoselective synthesis of vinyl ethers as well as in carbon homologation of aldehydes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry Central Journal
化学-化学综合
CiteScore
4.40
自引率
0.00%
发文量
0
审稿时长
3.5 months
期刊介绍:
BMC Chemistry is an open access, peer reviewed journal that considers all articles in the broad field of chemistry, including research on fundamental concepts, new developments and the application of chemical sciences to broad range of research fields, industry, and other disciplines. It provides an inclusive platform for the dissemination and discussion of chemistry to aid the advancement of all areas of research.
Sections:
-Analytical Chemistry
-Organic Chemistry
-Environmental and Energy Chemistry
-Agricultural and Food Chemistry
-Inorganic Chemistry
-Medicinal Chemistry
-Physical Chemistry
-Materials and Macromolecular Chemistry
-Green and Sustainable Chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


