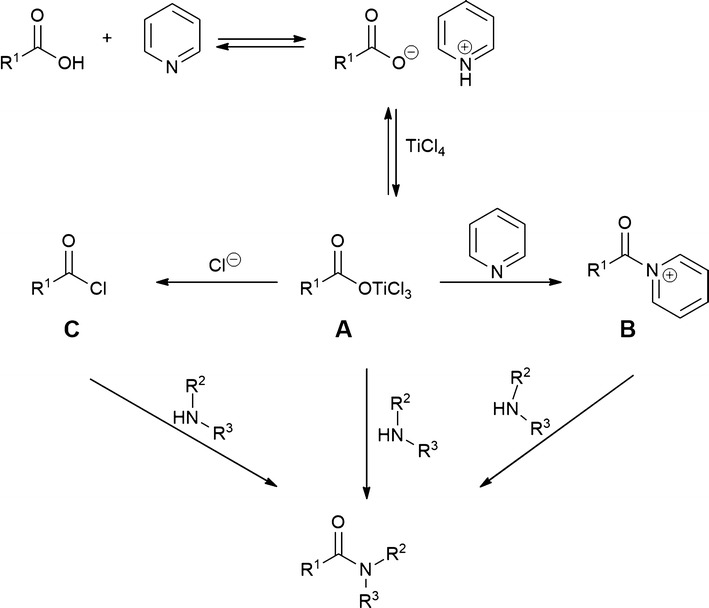
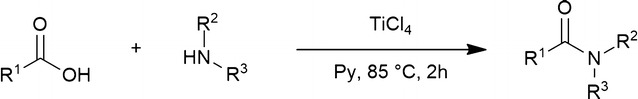
酰胺的形成:TiCl4介导的羧酸和胺的一锅缩合反应。
Q1 Chemistry
引用次数: 31
摘要
本文报道了在二氧化钛的存在下,羧酸和胺直接缩合合成酰胺的一般方法。在85°C的吡啶中进行酰胺化反应,广泛的底物提供了相应的酰胺产品,收率中等至优异,纯度高。当羧酸和胺都受到位阻时,反应的产率较低。该过程几乎完全保留了手性底物的立体化学完整性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Formation of amides: one-pot condensation of carboxylic acids and amines mediated by TiCl4.
A general procedure for the synthesis of amides via the direct condensation of carboxylic acids and amines in the presence of TiCl4 is reported. The amidation reaction was performed in pyridine at 85 °C with a wide range of substrates providing the corresponding amide products in moderate to excellent yields and high purity. The reaction proceeds with low yields when both the carboxylic acid and the amine are sterically hindered. The process takes place with nearly complete preservation of the stereochemical integrity of chiral substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry Central Journal
化学-化学综合
CiteScore
4.40
自引率
0.00%
发文量
0
审稿时长
3.5 months
期刊介绍:
BMC Chemistry is an open access, peer reviewed journal that considers all articles in the broad field of chemistry, including research on fundamental concepts, new developments and the application of chemical sciences to broad range of research fields, industry, and other disciplines. It provides an inclusive platform for the dissemination and discussion of chemistry to aid the advancement of all areas of research.
Sections:
-Analytical Chemistry
-Organic Chemistry
-Environmental and Energy Chemistry
-Agricultural and Food Chemistry
-Inorganic Chemistry
-Medicinal Chemistry
-Physical Chemistry
-Materials and Macromolecular Chemistry
-Green and Sustainable Chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


