胰高血糖素样肽-1受体激动剂Semaglutide在现实生活中的有效性和安全性:维护性渐进式血液透析患者的病例系列。
IF 0.9
Q4 UROLOGY & NEPHROLOGY
Case Reports in Nephrology and Dialysis
Pub Date : 2022-11-22
eCollection Date: 2022-09-01
DOI:10.1159/000527919
引用次数: 2
摘要
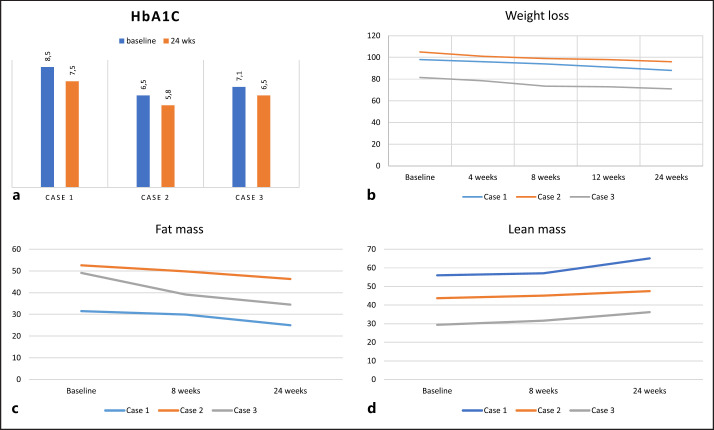
胰高血糖素样肽-1受体激动剂(GLP-1RA)是治疗2型糖尿病和减缓糖尿病肾病(DKD)进展的最新治疗选择之一。皮下(SC) semaglutide (Ozempic®)是一种GLP-1RA,半衰期延长约为1周。GLP-1RA在改善血糖控制方面非常有效,还显示出其他有益效果,如增加尿钠;降低血压和蛋白尿;减少氧化应激和炎症;延缓胃排空,抑制食欲;后者可能会导致显著的体重减轻。GLP-1RA可用于晚期CKD患者;欧洲药品管理局已批准使用所有市售的人GLP-1类似物,最低eGFR为15 mL/min/1.73 m2。然而,关于这些药物在肾脏替代治疗中的安全性和使用的研究很少。因此,在此,我们报告了3例晚期DKD患者每周1次的维持性渐进式血液透析,以描述SC西马鲁肽治疗的有效性和安全性,以及在西班牙一家医院血液透析部门6个月的随访中对血糖控制、降低HbA1c、蛋白尿、体重、血压控制和保留残余肾功能(RKF)的有利作用。这些作用可以改善发病率和死亡率,也可以预防蛋白尿和保存RKF。这可能使我们的患者维持每周一次的血液透析疗程,并可能促进他们列入肾移植等待名单。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficacy and Safety of Semaglutide, a Glucagon-Like Peptide-1 Receptor Agonist in Real-Life: A Case Series of Patients in Maintenance Incremental Hemodialysis.
The glucagon-like peptide-1 receptor agonists (GLP-1RA) are among the newest treatment options available for managing of type 2 diabetes mellitus and slowing the progression of diabetes kidney disease (DKD). Subcutaneous (SC) semaglutide (Ozempic®) is a GLP-1RA with an extended half-life of approximately 1 week. GLP-1RA are highly effective in improving glycemic control and also show other beneficial effects such as increased natriuresis; decreased blood pressure and albuminuria; reduction of oxidative stress and inflammation; delay of gastric emptying and suppress appetite; the latter may result in significant weight loss. GLP-1RA can be used in patients with advanced-stage CKD; the European Medicines Agency has approved the use of all commercially available human GLP-1 analogs up to a minimal eGFR of 15 mL/min/1.73 m2. However, studies of safety and use of these agents in renal replacement therapy are scarce. Therefore, herein we present 3 cases of patients with advanced DKD in maintenance incremental hemodialysis with 1 session per week to describe the efficacy and safety of the SC semaglutide treatment and the favorable effects on glycemic control, lowering HbA1c, albuminuria, weight, blood pressure control, and preservation of residual kidney function (RKF) during a 6-month follow-up in a hospital hemodialysis unit in Spain. These effects could produce an improvement in morbidity and mortality and could also prevent albuminuria and preserve the RKF. This may allow our patients to maintain a weekly hemodialysis session and could facilitate their inclusion in the kidney transplant waiting lists.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Case Reports in Nephrology and Dialysis
UROLOGY & NEPHROLOGY-
CiteScore
1.20
自引率
0.00%
发文量
36
审稿时长
10 weeks
期刊介绍:
This peer-reviewed online-only journal publishes original case reports covering the entire spectrum of nephrology and dialysis, including genetic susceptibility, clinical presentation, diagnosis, treatment or prevention, toxicities of therapy, critical care, supportive care, quality-of-life and survival issues. The journal will also accept case reports dealing with the use of novel technologies, both in the arena of diagnosis and treatment. Supplementary material is welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


