b细胞对肌肉注射二价病毒样颗粒人诺如病毒疫苗的反应。
Q2 Biochemistry, Genetics and Molecular Biology
Clinical and Vaccine Immunology
Pub Date : 2017-05-05
Print Date: 2017-05-01
DOI:10.1128/CVI.00571-16
引用次数: 19
摘要
人类诺如病毒(HuNoVs)是世界范围内急性胃肠炎的主要病因。一种病毒样颗粒(VLP)候选疫苗诱导血清组织-血型抗原(HBGA)阻断抗体的产生,这是首次发现的与HuNoV胃肠炎保护相关的抗体。最近,病毒特异性IgG记忆B细胞被确定为另一个潜在的相关保护对HuNoV胃肠炎。我们评估了肌肉注射二价(基因组I,基因型1 [GI])后b细胞的反应。1]/基因组II,基因型4 [GII.4]) VLP疫苗使用的方案与用于评估实验性gii .1 HuNoV感染后细胞免疫的方案相同。对G1.1感染的细胞免疫动力学和强度与接种VLP后进行了比较。用二价VLP疫苗肌内免疫可诱导抗体分泌细胞(ASCs)和记忆B细胞的产生。ASC反应在第一次接种疫苗后第7天达到高峰,并在第28天恢复到接近基线水平。在第28天接种第二剂疫苗后,ASCs的增加幅度最小。抗原特异性IgG记忆B细胞在接种GI.1和gi .4 VLPs后180天仍然存在。b细胞对疫苗接种反应的总体趋势与对感染反应的趋势相似,其中ASC对IgA的反应和记忆b细胞对IgG的反应有更大的偏倚。ASC和记忆b细胞对疫苗GI.1 VLP成分的反应程度也与GI.1感染后的反应相当。IgG记忆B细胞的产生和在180天的持久性是一个关键的发现,并强调了未来研究确定IgG记忆B细胞是否与疫苗接种后的保护相关的必要性。(本研究已在ClinicalTrials.gov注册,注册号为:NCT01168401)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
B-Cell Responses to Intramuscular Administration of a Bivalent Virus-Like Particle Human Norovirus Vaccine.
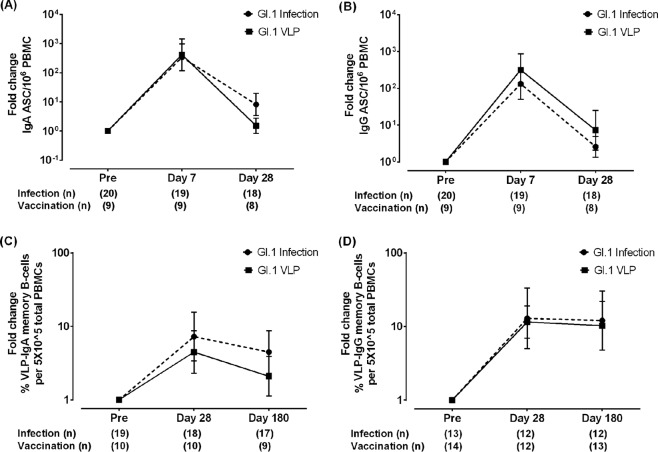
ABSTRACT Human noroviruses (HuNoVs) are a leading cause of acute gastroenteritis worldwide. A virus-like particle (VLP) candidate vaccine induces the production of serum histo-blood group antigen (HBGA)-blocking antibodies, the first identified correlate of protection from HuNoV gastroenteritis. Recently, virus-specific IgG memory B cells were identified to be another potential correlate of protection against HuNoV gastroenteritis. We assessed B-cell responses following intramuscular administration of a bivalent (genogroup I, genotype 1 [GI.1]/genogroup II, genotype 4 [GII.4]) VLP vaccine using protocols identical to those used to evaluate cellular immunity following experimental GI.1 HuNoV infection. The kinetics and magnitude of cellular immunity to G1.1 infection were compared to those after VLP vaccination. Intramuscular immunization with the bivalent VLP vaccine induced the production of antibody-secreting cells (ASCs) and memory B cells. ASC responses peaked at day 7 after the first dose of vaccine and returned to nearly baseline levels by day 28. Minimal increases in ASCs were seen after a second vaccine dose at day 28. Antigen-specific IgG memory B cells persisted at day 180 postvaccination for both GI.1 and GII.4 VLPs. The overall trends in B-cell responses to vaccination were similar to the trends in the responses to infection, where there was a greater bias of an ASC response toward IgA and a memory B-cell response to IgG. The magnitude of the ASC and memory B-cell responses to the GI.1 VLP component of the vaccine was also comparable to that of the responses following GI.1 infection. The production of IgG memory B cells and persistence at day 180 is a key finding and underscores the need for future studies to determine if IgG memory B cells are a correlate of protection following vaccination. (This study has been registered at ClinicalTrials.gov under registration no. NCT01168401.)
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Clinical and Vaccine Immunology
医学-传染病学
CiteScore
2.88
自引率
0.00%
发文量
0
审稿时长
1.5 months
期刊介绍:
Cessation. First launched as Clinical and Diagnostic Laboratory Immunology (CDLI) in 1994, CVI published articles that enhanced the understanding of the immune response in health and disease and after vaccination by showcasing discoveries in clinical, laboratory, and vaccine immunology. CVI was committed to advancing all aspects of vaccine research and immunization, including discovery of new vaccine antigens and vaccine design, development and evaluation of vaccines in animal models and in humans, characterization of immune responses and mechanisms of vaccine action, controlled challenge studies to assess vaccine efficacy, study of vaccine vectors, adjuvants, and immunomodulators, immune correlates of protection, and clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


