苯-1,2,4-三唑作为cb1大麻素受体配体的制备及体外药理评价。
International Journal of Medicinal Chemistry
Pub Date : 2016-01-01
Epub Date: 2016-03-31
DOI:10.1155/2016/1257098
引用次数: 1
摘要
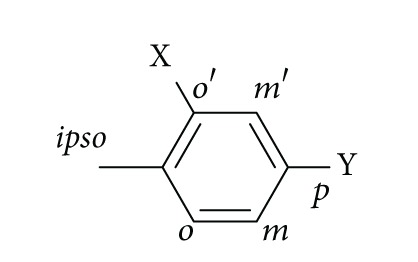
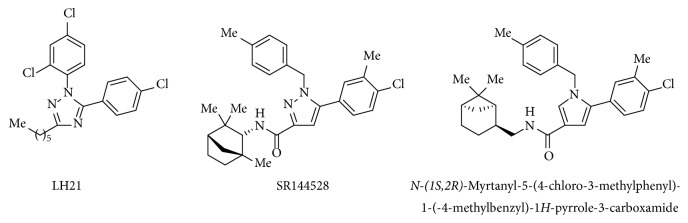
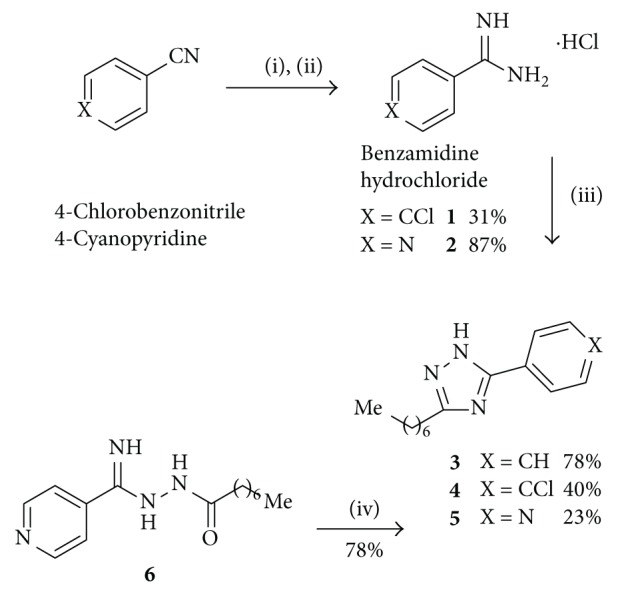
在之前的研究中,我们已经确定了3-烷基-1,5-二烷基- 1h -1,2,4-三唑是一类新的大麻素1型受体(CB1R)拮抗剂。为了扩大以1,2,4-三唑为中心支架的大麻素配体的数量,我们合成了一系列新的1-苄基- 1h -1,2,4-三唑,并通过CB1R放射配体结合试验对其中一些进行了评价。化合物12a显示出最有趣的药理特性,在纳摩尔范围内具有CB1R亲和力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Benzyl-1,2,4-triazoles as CB 1 Cannabinoid Receptor Ligands: Preparation and In Vitro Pharmacological Evaluation.
In a previous study, we have identified 3-alkyl-1,5-diaryl-1H-1,2,4-triazoles to be a novel class of cannabinoid type 1 receptor (CB1R) antagonists. In order to expand the number of cannabinoid ligands with a central 1,2,4-triazole scaffold, we have synthesized a novel series of 1-benzyl-1H-1,2,4-triazoles, and some of them were evaluated by CB1R radioligand binding assays. Compound 12a showed the most interesting pharmacological properties, possessing a CB1R affinity in the nanomolar range.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Medicinal Chemistry
CHEMISTRY, MEDICINAL-
自引率
0.00%
发文量
0
期刊介绍:
International Journal of Medicinal Chemistry is a peer-reviewed, Open Access journal that publishes original research articles as well as review articles in all areas of chemistry associated with drug discovery, design, and synthesis. International Journal of Medicinal Chemistry is a peer-reviewed, Open Access journal that publishes original research articles as well as review articles in all areas of chemistry associated with drug discovery, design, and synthesis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


