雷马唑仑致过敏反应后心脏骤停1例报告。
IF 0.5
引用次数: 8
摘要
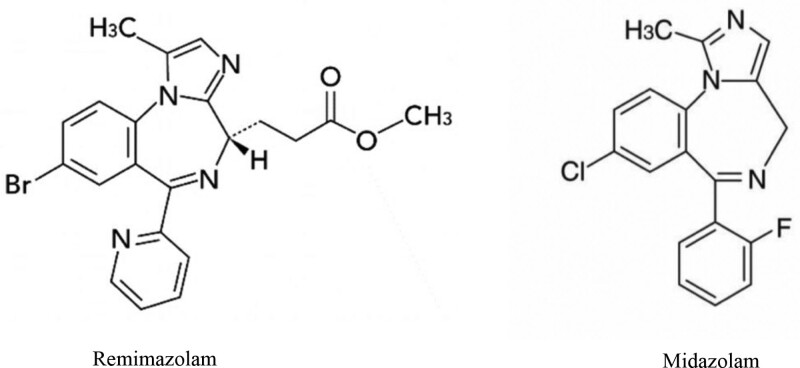
雷马唑仑是最近批准的苯二氮卓类镇静剂。我们报告一例72岁的男子谁经历了心脏骤停,由于严重的过敏反应后立即全身麻醉诱导。根据皮肤试验的结果,包括对葡聚糖40(雷马唑仑溶液中的一种赋形剂)的试验结果,以及对三种麻醉期间给药的审查,确定雷马唑仑为可能的病原体。虽然雷马唑仑在结构上与咪达唑仑相似,但该患者在过敏反应前后均未对咪达唑仑过敏。本报告强调了对雷马唑仑过敏反应的潜在风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cardiac Arrest Following Remimazolam-Induced Anaphylaxis: A Case Report.
Remimazolam is a recently approved benzodiazepine sedative. We report a case of a 72-year-old man who experienced a cardiac arrest due to severe anaphylaxis immediately after general anesthesia induction. Based on the results of skin tests, including those for dextran 40, an excipient in the remimazolam solution, and a review of drugs given during 3 anesthetics, remimazolam was identified as the probable causative agent. Although remimazolam is structurally similar to midazolam, the patient was not allergic to midazolam as demonstrated before and after anaphylaxis. This report highlights the potential risk of allergic reactions to remimazolam.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

A&A Practice
ANESTHESIOLOGY-
自引率
0.00%
发文量
0
期刊介绍:
A & A Case Reports, our new online journal publishing Case Reports, related Editorial Commentary, and Correspondence. Anesthesia & Analgesia 1 and Anesthesiology 2 recently announced that they were suspending publication of Case Reports. One reason is that Case Reports typically reduce the Impact Factor of a journal because they are rarely cited. Regardless of the merits of Impact Factor as a metric of journal worth, journals and their editors necessarily consider Impact Factor in strategic planning. At the same time, Case Reports are appreciated by readers for describing “real life” management of difficult or unusual cases not often encountered by practitioners. In a recent issue of Anesthesia & Analgesia, Steven Shafer1 identified many Case Reports whose publication launched productive careers dedicated to solving the puzzle posed by an unusual observation in a single patient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


