新型芳基酮类P1-P3连接大环BACE-1抑制剂的设计与合成
Q2 Pharmacology, Toxicology and Pharmaceutics
Open Medicinal Chemistry Journal
Pub Date : 2015-03-31
eCollection Date: 2015-01-01
DOI:10.2174/1874104501509010013
引用次数: 1
摘要
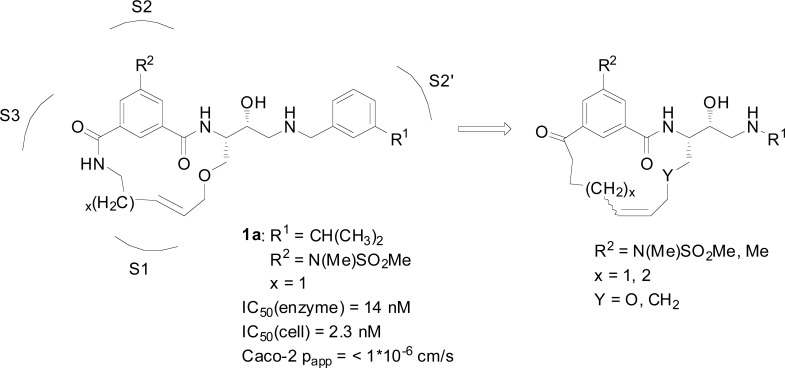
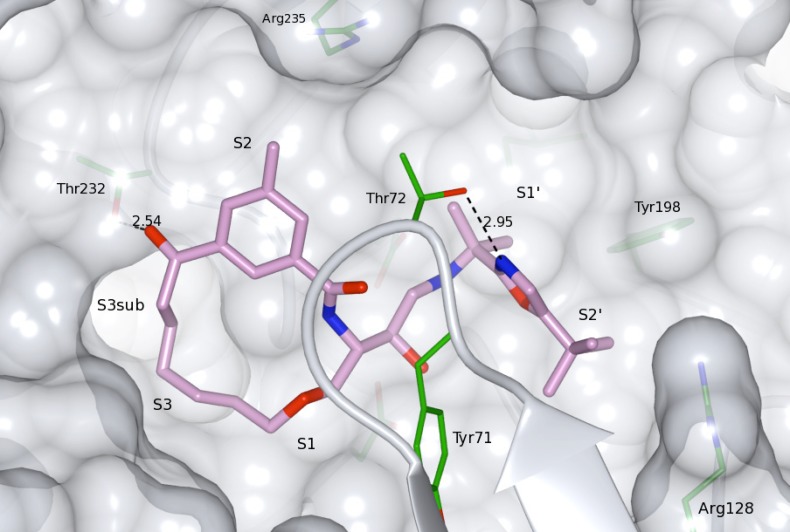
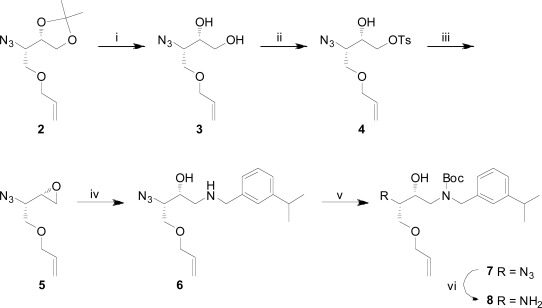
设计、合成了一系列含芳基酮P1-P3连接的大环BACE-1抑制剂,并与先前已知和广泛研究的相应P2异眼酰胺片段的化合物进行了比较,目的是在保持酶和细胞活性的同时提高其通透性。与早期合成的抑制剂相比,一些抑制剂显示出Caco-2细胞基通透性的显著增加,特别是保留了活性,表明这种方法可能产生性能更好的BACE-1抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design and Synthesis of Novel Arylketo-containing P1-P3 Linked Macro-cyclic BACE-1 Inhibitors.
A series of arylketo-containing P1-P3 linked macrocyclic BACE-1 inhibitors were designed, synthesized, and compared with compounds with a previously known and extensively studied corresponding P2 isophthalamide moiety with the aim to improve on permeability whilst retaining the enzyme- and cell-based activities. Several inhibitors displayed substantial increases in Caco-2 cell-based permeability compared to earlier synthesized inhibitors and notably also with retained activities, showing that this approach might yield BACE-1 inhibitors with improved properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Open Medicinal Chemistry Journal
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
4.40
自引率
0.00%
发文量
4
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


