铬吡啶酮的合成和体外细胞毒性活性。
International Journal of Medicinal Chemistry
Pub Date : 2013-01-01
Epub Date: 2012-01-08
DOI:10.1155/2013/984329
引用次数: 0
摘要
研究人员合成了新型取代的铬吡啶酮类化合物(3a-j 和 6a-d),并在体外评估了这些化合物对前列腺癌(PC-3)、乳腺癌(MCF-7)、中枢神经系统癌症(IMR-32)、宫颈癌(Hela)和肝癌(Hep-G2)等多种人类癌细胞株的细胞毒性活性。特别是与标准药物相比,含有烯丙基的化合物 6b 显示出显著的细胞毒性潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
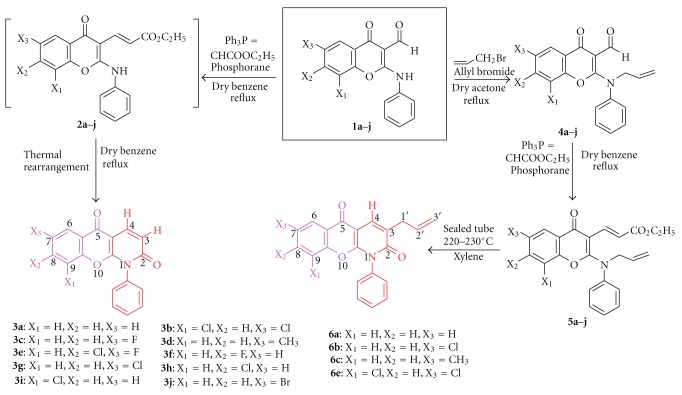
Synthesis and in vitro cytotoxic activity of chromenopyridones.
Novel substituted chromenopyridones (3a-j and 6a-d) were synthesized and evaluated in vitro for the cytotoxic activity against various human cancer cell lines such as prostate (PC-3), breast (MCF-7), CNS (IMR-32), cervix (Hela), and liver (Hep-G2). preliminary cytotoxic screening showed that all the compounds possess a good to moderate inhibitory activity against various cancer cell lines. Particularly, compound 6b bearing allyl moiety displayed a significant cytotoxic potential in comparison to standard drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Medicinal Chemistry
CHEMISTRY, MEDICINAL-
自引率
0.00%
发文量
0
期刊介绍:
International Journal of Medicinal Chemistry is a peer-reviewed, Open Access journal that publishes original research articles as well as review articles in all areas of chemistry associated with drug discovery, design, and synthesis. International Journal of Medicinal Chemistry is a peer-reviewed, Open Access journal that publishes original research articles as well as review articles in all areas of chemistry associated with drug discovery, design, and synthesis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


