巨型芽孢杆菌四吡咯生物合成酶卟啉原脱氨酶的结晶和初步 X 射线表征。
IF 0.9
4区 生物学
Acta Crystallographica Section F-structural Biology and Crystallization Communications
Pub Date : 2013-08-01
Epub Date: 2013-07-27
DOI:10.1107/S1744309113018526
引用次数: 0
摘要
卟啉原脱氨酶(PBGD;羟甲基比兰合成酶;EC 2.5.1.61)催化四吡咯生物合成途径的早期步骤,在该步骤中,四分子单吡咯卟啉原缩合成线性四吡咯。该酶具有一个二吡咯烷辅助因子,该辅助因子通过硫醚桥与一个不变的半胱氨酸残基共价连接。在大肠杆菌中表达 His 标记形式的巨型芽孢杆菌 PBGD 使该物种的酶得以结晶并进行了初步的高分辨率 X 射线分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
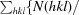
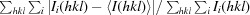
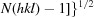
Crystallization and preliminary X-ray characterization of the tetrapyrrole-biosynthetic enzyme porphobilinogen deaminase from Bacillus megaterium.
The enzyme porphobilinogen deaminase (PBGD; hydroxymethylbilane synthase; EC 2.5.1.61) catalyses an early step of the tetrapyrrole-biosynthesis pathway in which four molecules of the monopyrrole porphobilinogen are condensed to form a linear tetrapyrrole. The enzyme possesses a dipyrromethane cofactor which is covalently linked by a thioether bridge to an invariant cysteine residue. Expression in Escherichia coli of a His-tagged form of Bacillus megaterium PBGD permitted the crystallization and preliminary X-ray analysis of the enzyme from this species at high resolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2-4 weeks
期刊介绍:
Acta Crystallographica Section F is a rapid structural biology communications journal.
Articles on any aspect of structural biology, including structures determined using high-throughput methods or from iterative studies such as those used in the pharmaceutical industry, are welcomed by the journal.
The journal offers the option of open access, and all communications benefit from unlimited free use of colour illustrations and no page charges. Authors are encouraged to submit multimedia content for publication with their articles.
Acta Cryst. F has a dedicated online tool called publBio that is designed to make the preparation and submission of articles easier for authors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


