含扩展P1取代基的羟乙基BACE-1抑制剂的设计与合成
Q2 Pharmacology, Toxicology and Pharmaceutics
Open Medicinal Chemistry Journal
Pub Date : 2013-03-08
Print Date: 2013-01-01
DOI:10.2174/1874104501307010001
引用次数: 28
摘要
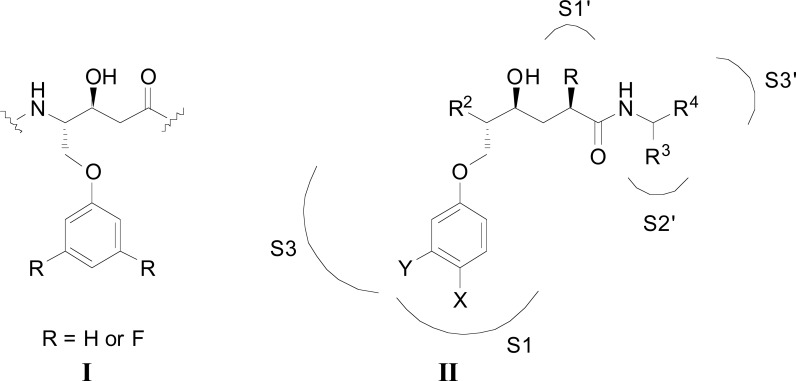
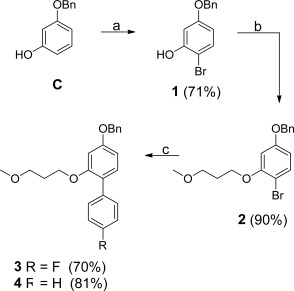
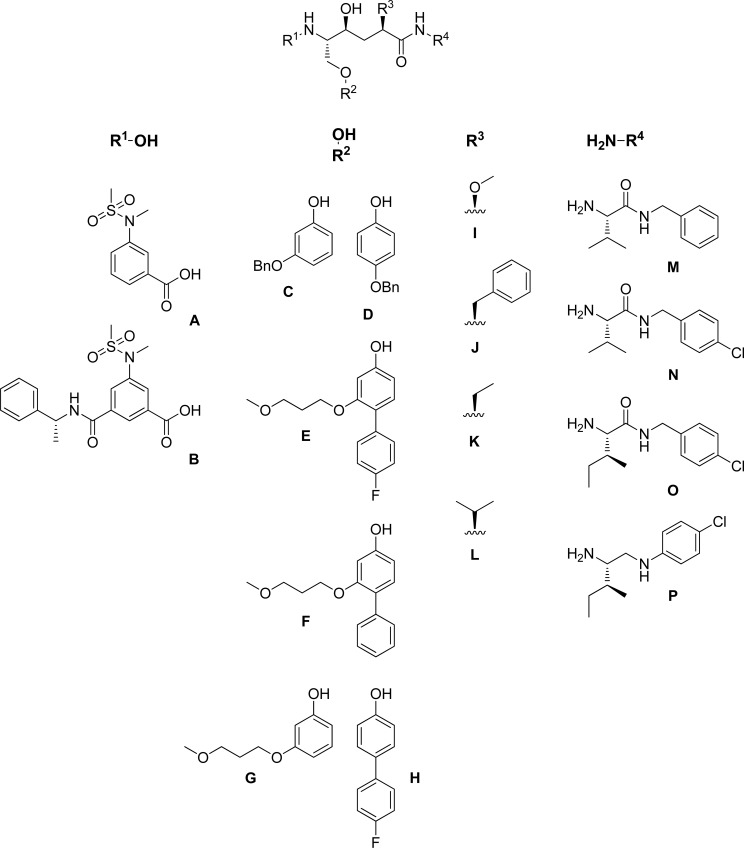
以羟基乙烯为中心核的新型BACE-1抑制剂已经被开发出来。加入修饰的P1´和扩展的P1´取代基,目的是分别探索与BACE-1的S1´和S1- s3口袋的潜在相互作用。抑制剂的IC50值在纳摩尔范围内,即最有效的化合物为69 nM。还讨论了可能的抑制剂与酶的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design and Synthesis of Hydroxyethylene-Based BACE-1 Inhibitors Incorporating Extended P1 Substituents.
Novel BACE-1 inhibitors with a hydroxyethylene central core have been developed. Modified P1´ and extended P1 substituents were incorporated with the aim to explore potential interactions with the S1´ and the S1-S3 pocket, respectively, of BACE-1. Inhibitors were identified displaying IC50 values in the nanomolar range, i.e. 69 nM for the most potent compound. Possible inhibitor interactions with the enzyme are also discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Open Medicinal Chemistry Journal
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
4.40
自引率
0.00%
发文量
4
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


