β-arrestin-1是内皮素-1诱导的β-catenin信号的核转录调节因子
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 79
摘要
尽管β-catenin在肿瘤进展中具有重要的基本病理生理作用,但其最终转录输出的机制尚未部分阐明。在这里,我们报告了β-arrestin-1(β-arr1)是上皮性卵巢癌(EOC)中内皮素(ET)-1诱导的β-catenin信号转导的表观遗传调节因子。在 ET-1 激活 ET A 受体(ETAR)时,β-arr1 会增加其核转位并直接与 β-catenin 结合。这反过来又增强了 β-catenin 的核积累和转录活性,而表达不能进行核分布的突变体 β-arr1 则可阻止这种活性。β-arr1-β-catenin相互作用通过促进组蛋白去乙酰化酶1(HDAC1)解离和p300乙酰转移酶在这些启动子基因上的招募,控制β-catenin靶基因(如ET-1、Axin 2、基质金属蛋白酶2和细胞周期蛋白D1)的表达,导致细胞迁移、侵袭和上皮细胞向间质转化所需的H3和H4组蛋白乙酰化和基因转录增强。β-arr1沉默或突变体β-arr1以及β-catenin或p300沉默都会减弱这些效应,这证实核β-arr1形成了一个功能复合物,能够调节β-catenin驱动的侵袭行为中的表观遗传变化。在转移性人EOC的小鼠正位模型中,沉默β-arr1或突变体β-arr1的表达以及阻断ETAR可抑制转移。在人EOC组织中,β-arr1-β-catenin核复合物选择性地富集于β-catenin靶基因启动子,与肿瘤分级相关,证实了体内β-arr1-β-catenin与EOC进展过程中的特定基因直接相关。总之,我们的研究深入揭示了β-arr1介导的表观遗传机制如何控制β-catenin的活性,揭示了其促进转移的核功能所需的新成分。本文章由计算机程序翻译,如有差异,请以英文原文为准。
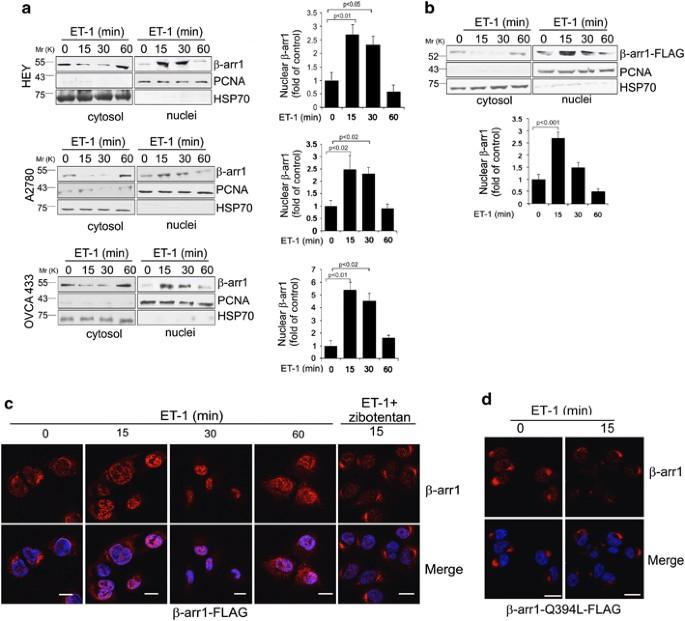
β-arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced β-catenin signaling
Despite the fundamental pathophysiological importance of β-catenin in tumor progression, the mechanism underlying its final transcriptional output has been partially elucidated. Here, we report that β-arrestin-1 (β-arr1) is an epigenetic regulator of endothelin (ET)-1-induced β-catenin signaling in epithelial ovarian cancer (EOC). In response to ET A receptor (ETAR) activation by ET-1, β-arr1 increases its nuclear translocation and direct binding to β-catenin. This in turn enhanced β-catenin nuclear accumulation and transcriptional activity, which was prevented by expressing a mutant β-arr1 incapable of nuclear distribution. β-arr1–β-catenin interaction controls β-catenin target gene expressions, such as ET-1, Axin 2, Matrix metalloproteinase 2, and Cyclin D1, by promoting histone deacetylase 1 (HDAC1) dissociation and the recruitment of p300 acetyltransferase on these promoter genes, resulting in enhanced H3 and H4 histone acetylation, and gene transcription, required for cell migration, invasion and epithelial-to-mesenchymal transition. These effects are abrogated by β-arr1 silencing or by mutant β-arr1, as well as by β-catenin or p300 silencing, confirming that nuclear β-arr1 forms a functional complex capable of regulating epigenetic changes in β-catenin-driven invasive behavior. In a murine orthotopic model of metastatic human EOC, silencing of β-arr1 or mutant β-arr1 expression, as well as ETAR blockade, inhibits metastasis. In human EOC tissues, β-arr1–β-catenin nuclear complexes are selectively enriched at β-catenin target gene promoters, correlating with tumor grade, confirming a direct in vivo β-arr1–β-catenin association at specific set of genes involved in EOC progression. Collectively, our study provides insights into how a β-arr1-mediated epigenetic mechanism controls β-catenin activity, unraveling new components required for its nuclear function in promoting metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


