使用微聚焦光束线从含有多个微结构域的蛔虫suum As-p18脂肪酸结合蛋白晶体中获得可用的衍射数据。
IF 0.9
4区 生物学
Acta Crystallographica Section F-structural Biology and Crystallization Communications
Pub Date : 2012-08-01
Epub Date: 2012-07-31
DOI:10.1107/S1744309112026553
引用次数: 2
摘要
As-p18是一种脂肪酸结合蛋白,来自于寄生线虫蛔虫。虽然它与哺乳动物细胞内脂肪酸结合蛋白的序列相似,但它含有线虫特有的特征。获得了晶体,但初始衍射数据分析显示它们是由许多“微畴”组成的。只能使用光束尺寸为12 × 8µm的微聚焦光束线收集可解释的数据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
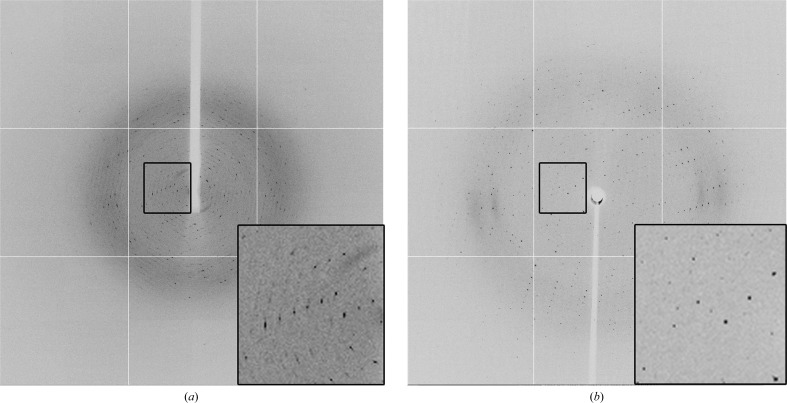
Useable diffraction data from a multiple microdomain-containing crystal of Ascaris suum As-p18 fatty-acid-binding protein using a microfocus beamline.
As-p18 is a fatty-acid-binding protein from the parasitic nematode Ascaris suum. Although it exhibits sequence similarity to mammalian intracellular fatty-acid-binding proteins, it contains features that are unique to nematodes. Crystals were obtained, but initial diffraction data analysis revealed that they were composed of a number of `microdomains'. Interpretable data could only be collected using a microfocus beamline with a beam size of 12 × 8 µm.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2-4 weeks
期刊介绍:
Acta Crystallographica Section F is a rapid structural biology communications journal.
Articles on any aspect of structural biology, including structures determined using high-throughput methods or from iterative studies such as those used in the pharmaceutical industry, are welcomed by the journal.
The journal offers the option of open access, and all communications benefit from unlimited free use of colour illustrations and no page charges. Authors are encouraged to submit multimedia content for publication with their articles.
Acta Cryst. F has a dedicated online tool called publBio that is designed to make the preparation and submission of articles easier for authors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


