方便、可扩展的 fmoc 保护多肽核酸骨架合成。
IF 1.3
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肽核酸骨架 Fmoc-AEG-OBn 是通过一种可扩展且具有成本效益的方法合成的。乙二胺经单叔丁氧羰基保护,然后用溴乙酸苄酯进行烷基化。Boc 基团被移除,取而代之的是 Fmoc 基团。合成从 50 克 Boc 酸酐开始,得到 31 克产品,总产率为 32%。Fmoc 保护的 PNA 主干是合成核碱基修饰 PNA 单体的关键中间体。因此,如果能更容易地获得这种分子,预计将有助于今后对核酸酶修饰的 PNA 的化学性质和应用进行研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
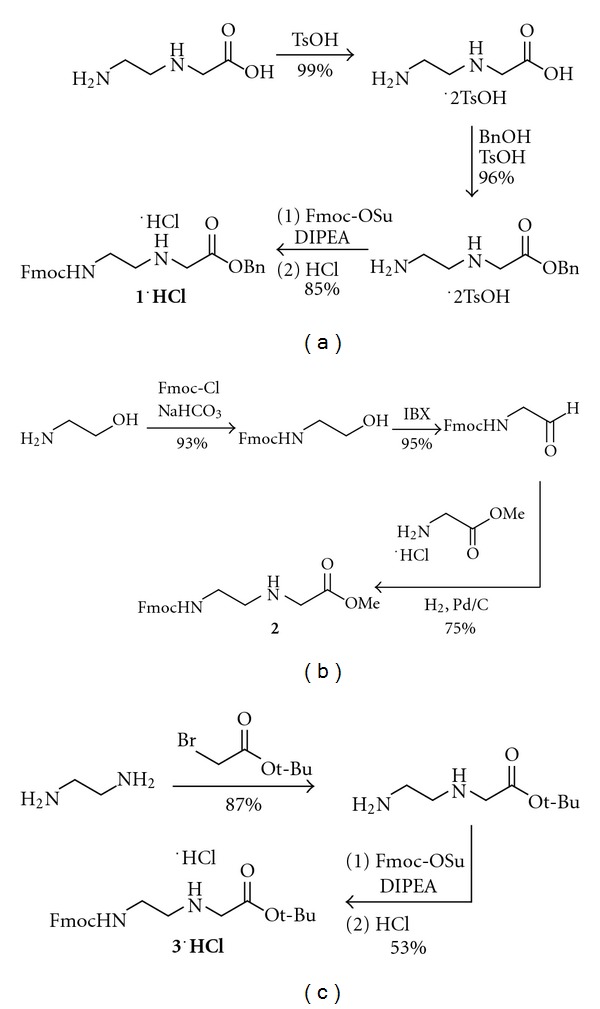
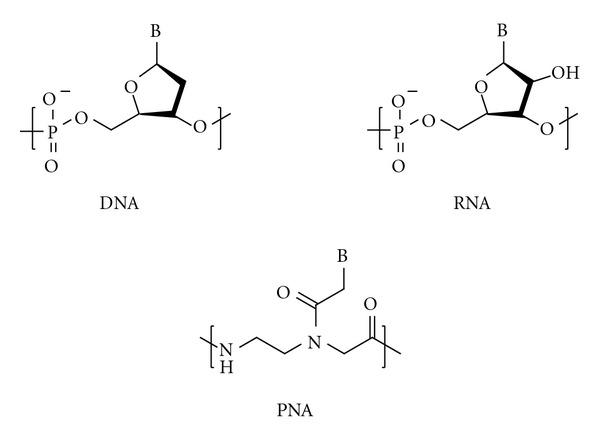
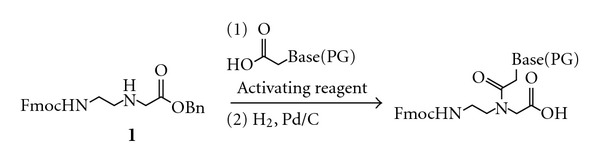
Convenient and scalable synthesis of fmoc-protected Peptide nucleic Acid backbone.
The peptide nucleic acid backbone Fmoc-AEG-OBn has been synthesized via a scalable and cost-effective route. Ethylenediamine is mono-Boc protected, then alkylated with benzyl bromoacetate. The Boc group is removed and replaced with an Fmoc group. The synthesis was performed starting with 50 g of Boc anhydride to give 31 g of product in 32% overall yield. The Fmoc-protected PNA backbone is a key intermediate in the synthesis of nucleobase-modified PNA monomers. Thus, improved access to this molecule is anticipated to facilitate future investigations into the chemical properties and applications of nucleobase-modified PNA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nucleic Acids
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
3.10
自引率
21.70%
发文量
5
审稿时长
12 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


