利用自动二维网格筛对 SHARPIN 结晶进行优化。
IF 0.9
4区 生物学
Acta Crystallographica Section F-structural Biology and Crystallization Communications
Pub Date : 2012-07-01
Epub Date: 2012-06-28
DOI:10.1107/S1744309112022208
引用次数: 0
摘要
人 SHARPIN 的 N 端片段在大肠杆菌中重组表达、纯化并结晶。通过使用二维网格筛一步优化种子稀释和蛋白质浓度,获得了适合 X 射线衍射的晶体。晶体属于原始四方空间群 P4(3)2(1)2,单位晶胞参数 a = b = 61.55,c = 222.81 Å。从 100 K 的原生和硒代蛋氨酸蛋白质晶体中收集了完整的数据集,分辨率分别为 2.6 和 2.0 Å。本文章由计算机程序翻译,如有差异,请以英文原文为准。
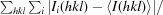

Crystallization of SHARPIN using an automated two-dimensional grid screen for optimization.
An N-terminal fragment of human SHARPIN was recombinantly expressed in Escherichia coli, purified and crystallized. Crystals suitable for X-ray diffraction were obtained by a one-step optimization of seed dilution and protein concentration using a two-dimensional grid screen. The crystals belonged to the primitive tetragonal space group P4(3)2(1)2, with unit-cell parameters a = b = 61.55, c = 222.81 Å. Complete data sets were collected from native and selenomethionine-substituted protein crystals at 100 K to 2.6 and 2.0 Å resolution, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2-4 weeks
期刊介绍:
Acta Crystallographica Section F is a rapid structural biology communications journal.
Articles on any aspect of structural biology, including structures determined using high-throughput methods or from iterative studies such as those used in the pharmaceutical industry, are welcomed by the journal.
The journal offers the option of open access, and all communications benefit from unlimited free use of colour illustrations and no page charges. Authors are encouraged to submit multimedia content for publication with their articles.
Acta Cryst. F has a dedicated online tool called publBio that is designed to make the preparation and submission of articles easier for authors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


