酸性介质中芳烃H/D交换的亲电RhI催化剂:亲电芳香取代机制的证据
IF 5.062
引用次数: 14
摘要
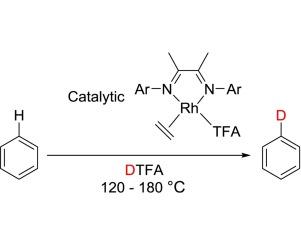
原位合成了一系列具有不同电子和位阻性质的双齿氮给体负载的新型铑(I)配合物,并对其催化芳烃C-H /D活化进行了评价。在三氟乙酸(HTFA)中,这些配合物被提出通过亲电芳香取代机制介导芳烃C-H /D键的H/D交换,该机制涉及rh介导的HTFA(或DTFA)的激活。DFT计算支持所提出的H/D交换反应途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrophilic RhI catalysts for arene H/D exchange in acidic media: Evidence for an electrophilic aromatic substitution mechanism
A series of new rhodium (I) complexes supported by bidentate nitrogen-donor ligands with varying electronic and steric properties were synthesized in situ and evaluated for catalytic arene C–H/D activation. In trifluoroacetic acid (HTFA), these complexes are proposed to mediate H/D exchange of arene C–H/D bonds by an electrophilic aromatic substitution mechanism that involves Rh-mediated activation of HTFA (or DTFA). DFT calculations support the proposed pathway for the H/D exchange reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2.8 months
期刊介绍:
The Journal of Molecular Catalysis A: Chemical publishes original, rigorous, and scholarly full papers that examine the molecular and atomic aspects of catalytic activation and reaction mechanisms in homogeneous catalysis, heterogeneous catalysis (including supported organometallic catalysis), and computational catalysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


