铜(II)芳基腙配合物对水中Henry反应中CH活化的催化作用
IF 5.062
引用次数: 48
摘要
以硝酸铜(II)和钠(Z)-2-(2-(1,3-二氧基-1-(苯胺)丁基-2-乙基)肼基)苯磺酸钠(NaH2L)为原料,在咪唑(im)(2)或吡嗪(py)(3)不存在的情况下合成了3种新的水溶性铜(II)配合物[Cu(HL)(H2O){(CH3)2NCHO}](1)、[Cu(HL)(CH3OH) 2(im)4]·CH3OH(2)和[Cu(HL)(CH3OH)]2(μ2-py)(3),并进行了表征。配合物1-3作为立体选择性CH活化催化剂,用于硝基烷与各种醛在水中的模型硝基醛(Henry)缩合反应。1是最活跃的催化剂,产率为64−87%,顺反选择性高达77:23。本文章由计算机程序翻译,如有差异,请以英文原文为准。
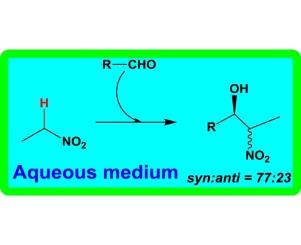
Copper(II) arylhydrazone complexes as catalysts for CH activation in the Henry reaction in water
Three new water-soluble copper(II) complexes [Cu(HL)(H2O){(CH3)2NCHO}] (1), [Cu(H2L)2(im)4]·CH3OH (2) and [Cu(HL)(CH3OH)]2(μ2-py) (3) were synthesized from copper(II) nitrate and sodium (Z)-2-(2-(1,3-dioxo-1-(phenylamino)butan-2-ylidene)hydrazinyl)benzene-sulfonate (NaH2L), in the absence (for 1) and presence of imidazole (im) (for 2) or pyrazine (py) (for 3), and fully characterized. The complexes 1–3 have been tested as stereoselective CH activating catalysts for the model nitroaldol (Henry) condensation of nitroethane with various aldehydes in water. 1 was the most active catalyst affording 64−87% yields with syn/anti diasteroselectivities up to 77:23.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2.8 months
期刊介绍:
The Journal of Molecular Catalysis A: Chemical publishes original, rigorous, and scholarly full papers that examine the molecular and atomic aspects of catalytic activation and reaction mechanisms in homogeneous catalysis, heterogeneous catalysis (including supported organometallic catalysis), and computational catalysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


