细胞因子标记物高灵敏度免疫分析技术的跨平台比较:创伤后应激障碍和帕金森病的平台表现
摘要
越来越多的证据表明,创伤后应激障碍(PTSD)和帕金森病(PD)存在全身性炎症,然而,这些假定的生物标志物的发现不一致且缺乏可重复性,阻碍了这一领域的进展。不同平台的表现差异可能导致生物标志物文献缺乏共识,正如在许多精神疾病(包括PTSD)中所看到的那样。因此,需要一种高性能、可扩展且经过验证的平台来发现和开发用于药物开发和临床诊断的炎症生物标志物。为了确定在未来生物标志物发现工作中使用的最佳平台,我们对五种领先的平台技术进行了全面的跨平台和交叉分析评估。这项初步评估集中在与PTSD有关的四种细胞因子——白细胞介素(IL)-1β、IL-6、肿瘤坏死因子(TNF)-α和干扰素(IFN)-γ。为了评估平台的性能并了解脑部疾病患者可能的测量结果,我们从PTSD (n = 13)或帕金森病患者(n = 14)以及健康对照(n = 5)中获取了血清和血浆样本。我们通过一些常见的分析参数比较了平台的性能,包括分析精度、灵敏度、内源性分析物检测频率(FEAD)、平台之间的相关性、和平行测量细胞因子使用一系列稀释系列。单分子阵列(Simoa™)超灵敏平台(Quanterix)、MESO V-Plex (Mesoscale Discovery)和Luminex xMAP®(Myriad)由各自的供应商进行检测,而Luminex®和Quantikine®高灵敏度ELISA检测由R&D System的生物标志物检测服务公司进行评估。Simoa™平台在所有分析物和临床人群中检测内源性分析物的灵敏度最高(即最高FEAD)。相比之下,MESO V-plex、R&D Luminex®和Quantikine®的性能变化更大,而Myriad的Luminex xMAP®在所有分析物和样品中都表现出较低的FEAD。Simoa™在检测内源性细胞因子方面也表现出很高的精度,这反映在<对于健康对照、PTSD患者和PD患者的样本,重复运行的方差系数(%CV)为20%。相比之下,MESO V-Plex、R&D Luminex®和Quantikine®在细胞因子的精确度方面表现不同。Myriad Luminex xMAP®不能包括在精度估计中,因为供应商没有重复运行样品。对于跨平台性能比较,IL-6的跨平台相关性最高,因此除了Myriad的Luminex xMAP®之外,所有平台在IL-6的测量中都具有很强的相关性(r范围= 0.59 - 0.86)。对于其他细胞因子,不同平台之间的相关性很低甚至没有相关性,因此报告的IL-1β、TNF-α和IFN-γ的测量值在不同的分析中有所不同。综上所述,这些发现提供了新的证据,表明免疫测定的选择可能会极大地影响报告的细胞因子发现。目前的研究提供了平台之间和免疫测定方法之间性能可变性的关键信息,这可能有助于在未来的研究中选择测定方法。此外,研究结果强调,随着新技术的出现,需要对免疫测定法进行比较评估,特别是在缺乏细胞因子定量评估的参考标准的情况下。
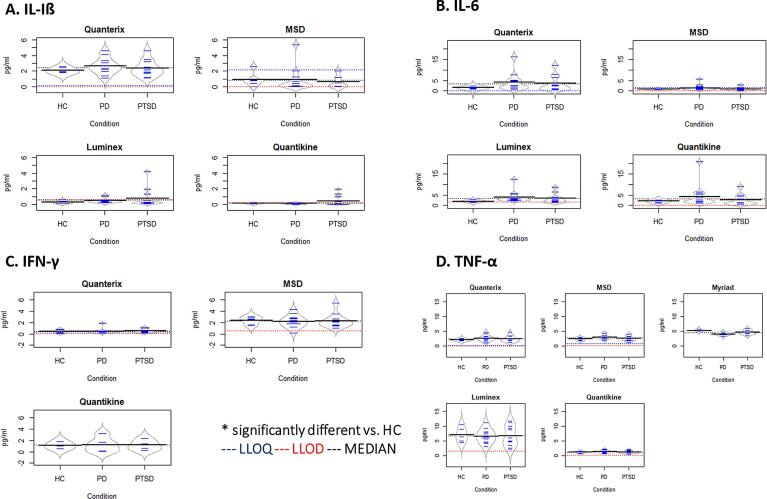

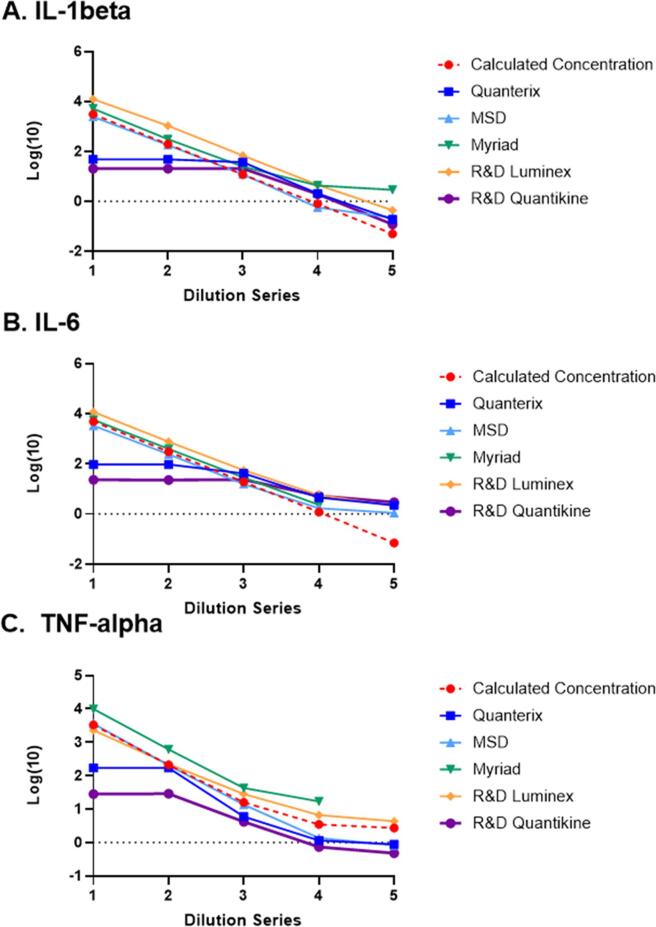
There is mounting evidence of systemic inflammation in post-traumatic stress disorder (PTSD) and Parkinson’s disease (PD), yet inconsistency and a lack of replicability in findings of putative biological markers have delayed progress in this space. Variability in performance between platforms may contribute to the lack of consensus in the biomarker literature, as has been seen for a number of psychiatric disorders, including PTSD. Thus, there is a need for high-performance, scalable, and validated platforms for the discovery and development of biomarkers of inflammation for use in drug development and as clinical diagnostics. To identify the best platform for use in future biomarker discovery efforts, we conducted a comprehensive cross-platform and cross-assay evaluation across five leading platform technologies. This initial assessment focused on four cytokines that have been implicated PTSD – interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ. To assess platform performance and understand likely measurements in individuals with brain disorders, serum and plasma samples were obtained from individuals with PTSD (n = 13) or Parkinson’s Disease (n = 14) as well as healthy controls (n = 5). We compared platform performance across a number of common analytic parameters, including assay precision, sensitivity, frequency of endogenous analyte detection (FEAD), correlation between platforms, and parallelism in measurement of cytokines using a serial dilution series. The single molecule array (Simoa™) ultra-sensitive platform (Quanterix), MESO V-Plex (Mesoscale Discovery), and Luminex xMAP® (Myriad) were conducted by their respective vendors, while Luminex® and Quantikine® high-sensitivity ELISA assays were evaluated by R&D System’s Biomarker Testing Services. The assay with the highest sensitivity in detecting endogenous analytes across all analytes and clinical populations (i.e. the highest FEAD), was the Simoa™ platform. In contrast, more variable performance was observed for MESO V-plex, R&D Luminex® and Quantikine®, while Myriad’s Luminex xMAP® exhibited low FEAD across all analytes and samples. Simoa™ also demonstrated high precision in detecting endogenous cytokines, as reflected in < 20 percent coefficient of variance (%CV) across replicate runs for samples from the healthy controls, PTSD patients, and PD patients. In contrast, MESO V-Plex, R&D Luminex® and Quantikine® had variable performance in terms of precision across cytokines. Myriad Luminex xMAP® could not be included in precision estimates because the vendor did not run samples in duplicate. For cross-platform performance comparisons, the highest cross-platform correlations were observed for IL-6 such that all platforms – except for Myriad’s Luminex xMAP® – had strong correlations with one another in measurements of IL-6 (r range = 0.59 – 0.86). For the other cytokines, there was low to no correlation across platforms, such that reported measurements of IL-1β, TNF-α, and IFN-γ varied across assays. Taken together, these findings provide novel evidence that the choice of immunoassay could greatly impact reported cytokine findings. The current study provides crucial information on the variability in performance between platforms and across immunoassays that may help inform the selection of assay in future research studies. Further, the results emphasize the need for performing comparative evaluations of immunoassays as new technologies emerge over time, particularly given the lack of reference standards for the quantitative assessments of cytokines.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


