重组腺相关病毒载体(rAAV2)介导的载脂蛋白B mrna特异性锤头核酶:一种自互补的AAV2载体提高了基因的表达。
摘要
背景:在人类中,载脂蛋白B (apoB)的过量产生与过早的冠状动脉疾病呈正相关。为了降低apoB mRNA的水平,我们设计了一种针对腺病毒介导的核苷酸序列GUA6679 (RB15)的apoB mRNA特异性锤头核酶,该酶能有效地切割并降低小鼠肝脏中80%的apoB mRNA,从而减轻了高脂症的病情。在本研究中,我们使用腺相关病毒载体血清2型(AAV2)和自互补的AAV2载体(scAAV2)来证明RB15长期组织特异性基因表达对体内apoB mRNA调控的影响。方法:利用AAV2载体(rAAV2-TTR-RB15)构建了由肝脏特异性甲状腺转甲状腺素(TTR)启动子驱动的锤头核酶RB15。HepG2细胞和高脂血症小鼠均缺乏低密度脂蛋白受体和apoB mRNA编辑酶基因(LDLR-/- apobec1 -/-;用rAAV2-TTR-RB15和对照载体raav - ttr - rb15突变体(失活核酶)转导LDb。在LDb小鼠转导后5个月测定核酶RB15对载脂蛋白代谢和动脉粥样硬化发展的影响。我们还设计了表达核酶RB15的自互补AAV2载体(scAAV2-TTR-RB15),并将其用于HepG2细胞的转导。研究旨在比较rAAV2-TTR-RB15和scAAV2-TTR-RB15的基因表达效率。结果:raav2 - trr -RB15转导后仅在第7天观察到核酶RB15 RNA降低HepG2细胞apoB mRNA水平的作用。并且,在rAAV2-TTR-RB15治疗5个月后,与对照载体rAAV2-TTR-RB15突变体治疗的LDb小鼠相比,LDb小鼠的apoB mRNA水平显著降低43%。此外,治疗后5个月小鼠肝脏中仍可检测到rAAV2-TTR-RB15病毒DNA和RB15核酶RNA。然而,该raav2 - trr -RB15载体在小鼠肝脏中介导了长时间但低水平的RB15核酶基因表达,并没有产生改变脂质水平或抑制动脉粥样硬化发展的治疗作用。相反,由scAAV2-TTR-RB15载体介导的核酶RB15 RNA在HepG2细胞转导后第1天立即表达。与对照载体scaav2 - ttr - rb15突变体相比,apoB mRNA水平降低了47% (p = 0.001)。结论:本研究证明rAAV2单链载体在小鼠肝脏中介导了长时间但不有效的转导。然而,scAAV2双链载体介导了肝细胞中快速有效的基因表达。这种使用scAAV2载体的策略代表了一种更好的表达小分子如核酶的方法。
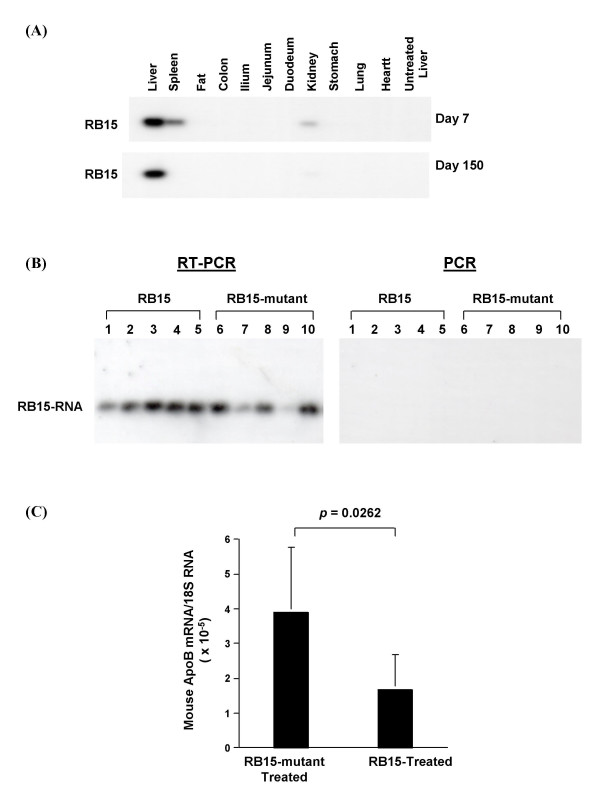
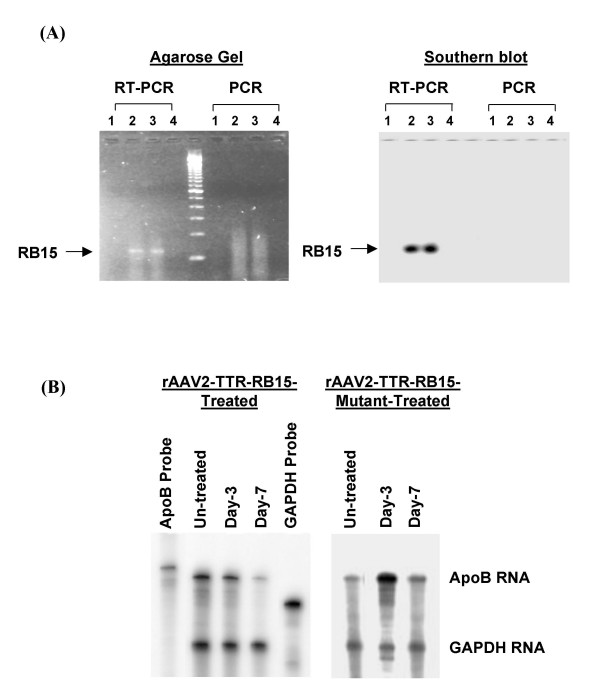

BACKGROUND: In humans, overproduction of apolipoprotein B (apoB) is positively associated with premature coronary artery diseases. To reduce the levels of apoB mRNA, we have designed an apoB mRNA-specific hammerhead ribozyme targeted at nucleotide sequences GUA6679 (RB15) mediated by adenovirus, which efficiently cleaves and decreases apoB mRNA by 80% in mouse liver and attenuates the hyperlipidemic condition. In the current study, we used an adeno-associated virus vector, serotype 2 (AAV2) and a self-complementary AAV2 vector (scAAV2) to demonstrate the effect of long-term tissue-specific gene expression of RB15 on the regulation apoB mRNA in vivo. METHODS: We constructed a hammerhead ribozyme RB15 driven by a liver-specific transthyretin (TTR) promoter using an AAV2 vector (rAAV2-TTR-RB15). HepG2 cells and hyperlipidemic mice deficient in both the low density lipoprotein receptor and the apoB mRNA editing enzyme genes (LDLR-/-Apobec1-/-; LDb) were transduced with rAAV2-TTR-RB15 and a control vector rAAV-TTR-RB15-mutant (inactive ribozyme). The effects of ribozyme RB15 on apoB metabolism and atherosclerosis development were determined in LDb mice at 5-month after transduction. A self-complementary AAV2 vector expressing ribozyme RB15 (scAAV2-TTR-RB15) was also engineered and used to transduce HepG2 cells. Studies were designed to compare the gene expression efficiency between rAAV2-TTR-RB15 and scAAV2-TTR-RB15. RESULTS: The effect of ribozyme RB15 RNA on reducing apoB mRNA levels in HepG2 cells was observed only on day-7 after rAAV2-TTR-RB15 transduction. And, at 5-month after rAAV2-TTR-RB15 treatment, the apoB mRNA levels in LDb mice were significantly decreased by 43%, compared to LDb mice treated with control vector rAAV2-TTR-RB15-mutant. Moreover, both the rAAV2-TTR-RB15 viral DNA and ribozyme RB15 RNA were still detectable in mice livers at 5-month after treatment. However, this rAAV2-TTR-RB15 vector mediated a prolonged but low level of ribozyme RB15 gene expression in the mice livers, which did not produce the therapeutic effects on alteration the lipid levels or the inhibition of atherosclerosis development. In contrast, the ribozyme RB15 RNA mediated by scAAV2-TTR-RB15 vector was expressed immediately at day-1 after transduction in HepG2 cells. The apoB mRNA levels were decreased 47% (p = 0.001), compared to the control vector scAAV2-TTR-RB15-mutant. CONCLUSION: This study provided evidence that the rAAV2 single-strand vector mediated a prolonged but not efficient transduction in mouse liver. However, the scAAV2 double-strand vector mediated a rapid and efficient gene expression in liver cells. This strategy using scAAV2 vectors represents a better approach to express small molecules such as ribozyme.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


