路易斯酸加速钯催化CH活化的研究
IF 5.062
引用次数: 11
摘要
在乙酰苯胺和尿素衍生物的定向邻烯基化和酰化反应中,证明了各种Lewis酸对钯催化的CH活化的加速作用。通过对不同钯催化剂、芳香底物中的导向基团和通用路易斯酸的研究,探讨了这种效应的普遍性。通过实验对反应进行了监测,并比较了不同类型路易斯酸的行为和活性。动力学研究揭示了一个速率决定CH活化的步骤,并通过DFT研究解释了Lewis酸对CH活化的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
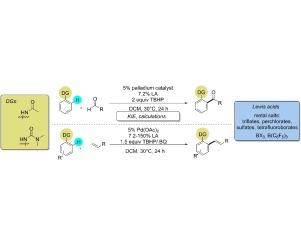
Study of Lewis acid accelerated palladium catalyzed CH activation
Acceleration of palladium catalyzed CH activation by various Lewis Acids was demonstrated on the directed ortho-alkenylation and acylation of acetanilide and urea derivatives. The universality of this effect was investigated by the study of different palladium catalysts, directing groups in the aromatic substrates and versatile Lewis acids. Experiments were carried out to monitor the reactions and to compare the behavior and activity of different types of Lewis acids. Kinetic investigation revealed a rate determining C
H activation step, and DFT studies were performed for the explanation of Lewis acid effect on C
H activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2.8 months
期刊介绍:
The Journal of Molecular Catalysis A: Chemical publishes original, rigorous, and scholarly full papers that examine the molecular and atomic aspects of catalytic activation and reaction mechanisms in homogeneous catalysis, heterogeneous catalysis (including supported organometallic catalysis), and computational catalysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


