烷烃的均相氧化:铑-烷基配合物的作用
IF 5.062
引用次数: 15
摘要
在全氟羧酸(CF3COOH, C3F7COOH)水溶液中,RhCl3-KI-NaCl和RhCl3-Cu (OAcf) 2-NaCl催化体系对烷烃和一氧化碳的双氧耦合氧化是有效的。在它们存在的情况下,主要是在过氧铑作为氧化剂的参与下,外球将烷烃氧化成相应的酯(醇)(机制A)。该过程部分地通过涉及rh -烷基中间体的内球机制B发生。机理B由(a)烷基氯化物的形成,(B)甲烷转化过程中醋酸的合成,以及(c)丙烷氧化过程中的位置选择性支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
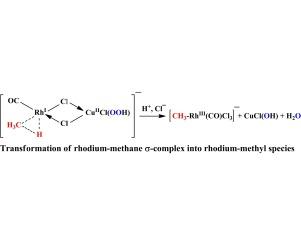
Homogeneous oxidation of alkanes: Role of rhodium–alkyl complexes
Catalytic systems RhCl3–KI–NaCl and RhCl3–Cu(OAcf)2–NaCl in aqueous perfluorinated carboxylic acids (CF3COOH, C3F7COOH) are effective in coupled oxidation of alkanes and carbon monoxide with dioxygen. In their presence, predominant is the outer-sphere oxidation of alkanes into respective esters (alcohols) with involvement of peroxo rhodium species as an oxidant (mechanism A). The process occurs partially by the inner-sphere mechanism B involving Rh–alkyl intermediates. Mechanism B is supported by (a) formation of alkyl chlorides, (b) synthesis of acetic acid in conversion of methane, and (c) positional selectivity in oxidation of propane.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2.8 months
期刊介绍:
The Journal of Molecular Catalysis A: Chemical publishes original, rigorous, and scholarly full papers that examine the molecular and atomic aspects of catalytic activation and reaction mechanisms in homogeneous catalysis, heterogeneous catalysis (including supported organometallic catalysis), and computational catalysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


