一个天然产物启发的含尿苷文库的快速合成
IF 3.784
3区 化学
Q1 Chemistry
引用次数: 0
摘要
采用液相平行合成方法制备天然产物激发的核苷类似物。利用点击化学和尿素或酰胺键形成,开发了含有炔和n保护氨基的关键中间体,以允许进一步的骨架和取代基多样性。采用固相萃取法实现快速纯化。该文库包含80个分子,包含两个多样性位置和一个手性中心,每个分子都能以良好的纯度和可接受的总收率有效地制备。还进行了细菌形态学研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
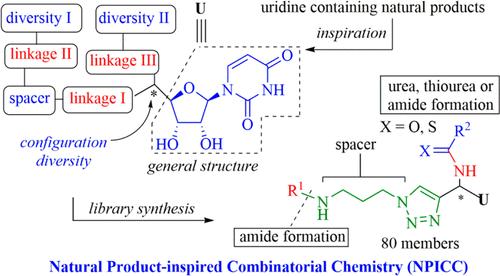
Rapid Synthesis of a Natural Product-Inspired Uridine Containing Library
The preparation of natural product-inspired nucleoside analogs using solution-phase parallel synthesis is described. The key intermediates containing alkyne and N-protected amino moieties were developed to allow for further skeleton and substituent diversity using click chemistry and urea or amide bond formation. Rapid purification was accomplished using solid-phase extraction. The obtained library comprised 80 molecules incorporating two diversity positions and one chiral center, each of which was efficiently prepared in good purity and acceptable overall yield. A bacterial morphology study was also performed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Combinatorial Science
CHEMISTRY, APPLIED-CHEMISTRY, MEDICINAL
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
The Journal of Combinatorial Chemistry has been relaunched as ACS Combinatorial Science under the leadership of new Editor-in-Chief M.G. Finn of The Scripps Research Institute. The journal features an expanded scope and will build upon the legacy of the Journal of Combinatorial Chemistry, a highly cited leader in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


