Azulene在食欲素/下丘脑分泌素受体激动剂中的联苯模拟物
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Azulene是药物中罕见的环状结构,我们研究了它是否可以在已知的食欲素受体激动剂Nag 26中作为联苯模拟物,Nag 26同时与食欲素受体OX1和OX2结合,并优先与OX2结合。在Ca2+升高实验中,最有效的azulene基化合物被鉴定为OX1食欲素受体激动剂(pEC50 = 5.79±0.07,最大反应= 81±8%(5个独立实验的s.e.m.)。然而,偶氮环和联苯支架在空间形状和电子分布上并不相同,它们的衍生物在结合位点上可能采用不同的结合方式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
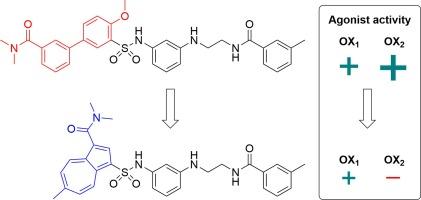
Azulene as a biphenyl mimetic in orexin/hypocretin receptor agonists
Azulene is a rare ring structure in drugs, and we investigated whether it could be used as a biphenyl mimetic in known orexin receptor agonist Nag 26, which is binding to both orexin receptors OX1 and OX2 with preference towards OX2. The most potent azulene-based compound was identified as an OX1 orexin receptor agonist (pEC50 = 5.79 ± 0.07, maximum response = 81 ± 8% (s.e.m. of five independent experiments) of the maximum response to orexin-A in Ca2+ elevation assay). However, the azulene ring and the biphenyl scaffold are not identical in their spatial shape and electron distribution, and their derivatives may adopt different binding modes in the binding site.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


